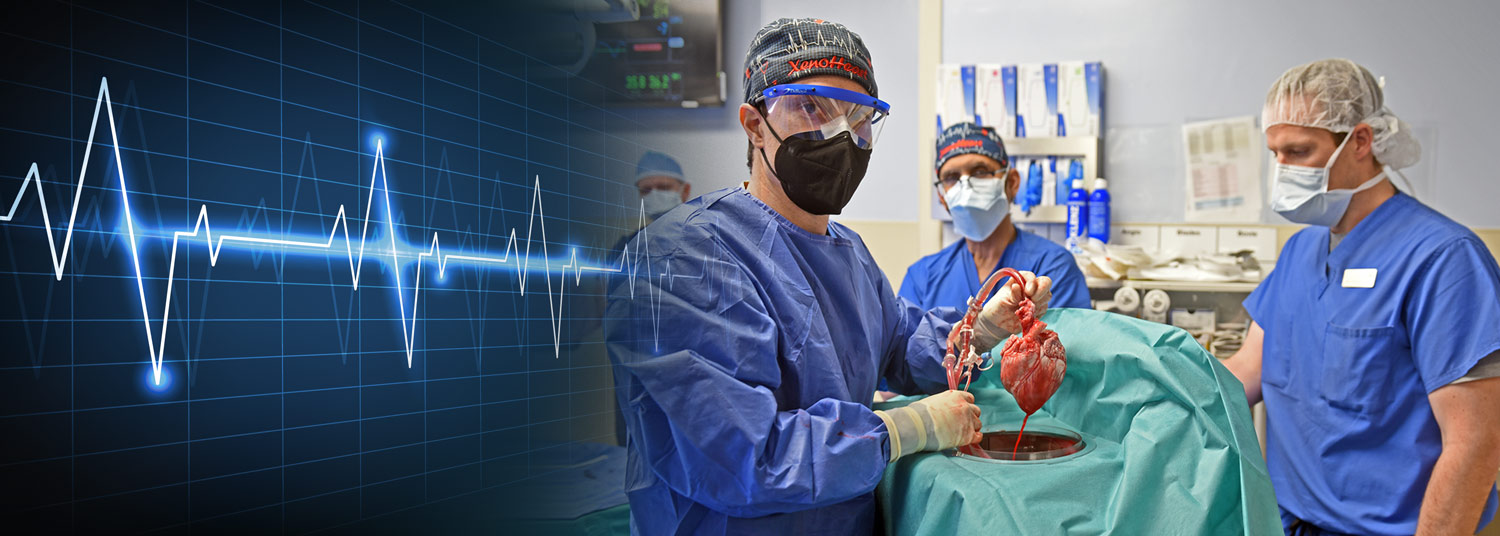
November 14, 2022 | Deborah Kotz
UMSOM Researchers Report on New Findings from Heart Monitoring Systems at American Heart Association Meeting
 Ten months after transplanting the first genetically-modified pig heart into a human patient, University of Maryland School of Medicine (UMSOM) researchers continue to report on new findings from the landmark transplant. Their latest study demonstrates for the first time that unexpected electrical changes occurred in the pig heart transplanted into the patient David Bennett. The findings were presented at the American Heart Association (AHA) meeting this past weekend.
Ten months after transplanting the first genetically-modified pig heart into a human patient, University of Maryland School of Medicine (UMSOM) researchers continue to report on new findings from the landmark transplant. Their latest study demonstrates for the first time that unexpected electrical changes occurred in the pig heart transplanted into the patient David Bennett. The findings were presented at the American Heart Association (AHA) meeting this past weekend.
The unexpected heart conduction measures, as seen on an electrocardiogram (ECG), did not contribute to the failure of the heart after two months but will help inform future cardiac xenotransplants.
“Electrical signals in a pig heart normally travel very quickly, faster than in a human heart, but we found that electrical signals in the transplanted pig heart traveled far more slowly in Mr. Bennett,” said study leader Timm Dickfeld, MD, PhD, professor of medicine and director of electrophysiology research at UMSOM. “At times, these signals traveled even more slowly than what we would expect from a human heart. To be clear, his heart rhythm was normal, it was only the time it takes for electricity to travel through the heart that was prolonged.”
He and his colleagues used an ECG to monitor the patient daily after his transplant. Monitoring the heart with ECG after transplantation is one way to assess the electrical conduction system after a heart transplant. A 12-lead ECG measures the electrical conduction in 12 different electrical angles of the heart.
 Specifically, the UMSOM researchers reviewed two ECG measures: the PR interval/QRS complex and the QT interval. The PR interval and QRS complex measure the time it takes electricity to travel from the top to the bottom chamber and across the bottom chambers, ultimately causing the heart to pump blood through the heart. The QT interval measures the time it takes the lower chambers of the heart to go through a full electrical cycle associated with a heartbeat.
Specifically, the UMSOM researchers reviewed two ECG measures: the PR interval/QRS complex and the QT interval. The PR interval and QRS complex measure the time it takes electricity to travel from the top to the bottom chamber and across the bottom chambers, ultimately causing the heart to pump blood through the heart. The QT interval measures the time it takes the lower chambers of the heart to go through a full electrical cycle associated with a heartbeat.
 “These findings do not appear to be associated with a pathological outcome like heart failure or signs of rejection,” said study co-author Bartley Griffith, MD, Professor of Surgery and The Thomas E. and Alice Marie Hales Distinguished Professor in Transplantation at UMSOM. He performed Mr. Bennett’s transplant surgery.
“These findings do not appear to be associated with a pathological outcome like heart failure or signs of rejection,” said study co-author Bartley Griffith, MD, Professor of Surgery and The Thomas E. and Alice Marie Hales Distinguished Professor in Transplantation at UMSOM. He performed Mr. Bennett’s transplant surgery.
“Genetic modifications made to the pig’s heart, to lessen the likelihood of immune system rejection, were not responsible for causing the unexpected EKG findings,” said study co-author Muhammad M. Mohiuddin, MD, Professor of Surgery and Scientific/Program Director of the Cardiac Xenotransplantation Program at UMSOM whose research led to this historic transplant.
 Other faculty members have analyzed heart imaging studies used to monitor heart function and check for signs of rejection of the transplant. Manjula Ananthram, MD, assistant professor of medicine at UMSOM, and her colleagues presented a research abstract at the heart association meeting showing that echocardiography worked well to measure the volume of blood pumped by the pig heart to the rest of the body when compared to the more invasive “gold standard” procedure, cardiac catheterization. “This was reassuring news and could eventually mean that we may not need to perform as much invasive monitoring of future patients who receive xenotransplants,” Dr. Ananthram said. “We are still carefully reviewing our data to determine the best way to monitor the function of the transplanted pig heart.”
Other faculty members have analyzed heart imaging studies used to monitor heart function and check for signs of rejection of the transplant. Manjula Ananthram, MD, assistant professor of medicine at UMSOM, and her colleagues presented a research abstract at the heart association meeting showing that echocardiography worked well to measure the volume of blood pumped by the pig heart to the rest of the body when compared to the more invasive “gold standard” procedure, cardiac catheterization. “This was reassuring news and could eventually mean that we may not need to perform as much invasive monitoring of future patients who receive xenotransplants,” Dr. Ananthram said. “We are still carefully reviewing our data to determine the best way to monitor the function of the transplanted pig heart.”
The historic xenotransplant surgery was conducted on January 7, 2022, by University of Maryland School of Medicine (UMSOM) faculty at the University of Maryland Medical Center (UMMC), together known as the University of Maryland Medicine. The surgery was the only available treatment option for the patient, David Bennett, who did not qualify for a traditional heart transplant and was in end-stage heart failure, nearing the end of his life. The procedure was authorized by the U.S. Food and Drug Administration under its expanded access (compassionate use) provision.
 Prior to the transplant, Mr. Bennett was bedridden for eight weeks with a life-threatening arrythmia and was connected to a heart-lung bypass machine, called extracorporeal membrane oxygenation (ECMO), to remain alive. Within days of the transplant, he was weaned from ECMO and participated in active rehabilitation for nearly two months. He visited regularly with family members and sang along to “America the Beautiful” while watching the Super Bowl in February with his physical therapist.
Prior to the transplant, Mr. Bennett was bedridden for eight weeks with a life-threatening arrythmia and was connected to a heart-lung bypass machine, called extracorporeal membrane oxygenation (ECMO), to remain alive. Within days of the transplant, he was weaned from ECMO and participated in active rehabilitation for nearly two months. He visited regularly with family members and sang along to “America the Beautiful” while watching the Super Bowl in February with his physical therapist.
The research team published its initial findings on the transplant last June in the New England Journal of Medicine.
“The transplanted pig heart was exposed to numerous variables in its new environment of the human body. One or several of them could have impacted the electrical conduction system,” said UMSOM Dean Mark T. Gladwin, MD, Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor. “We need to continue to examine the data and share what we learn with the medical community.”
Dr. Griffith was selected by the AHA to deliver the annual William W.L. Glenn Lecture at this year’s AHA meeting for his career-long achievements in cardiothoracic surgery. In addition to his recent accomplishments in advancing the field of cardiac xenotransplantation, he performed the first double lung transplant on a young man with cystic fibrosis in 1983. He was also one of the early pioneers testing the total artificial heart as a bridge device while waiting for donor heart to become available.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.3 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic, and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
Contact
Deborah Kotz
Senior Director of Media Relations
Office of Public Affairs & Communications
University of Maryland School of Medicine
Email: DKotz@som.umaryland.edu
o: 410-706-4255
c: 410-804-0054
t: @debkotz2
Related stories
Wednesday, January 08, 2025
Presenting a Path Forward for Future Genetically-Modified Pig Heart Transplants: Lessons Learned from Second Patient
Continuing significant advancements in the field of xenotransplantation, surgeon-scientists from the University of Maryland School of Medicine provided an extensive analysis on the second patient in the world to receive a genetically-modified pig organ. Lawrence Faucette, 58, received a pig heart at the University of Maryland Medical Center in 2023 to treat his end-stage heart failure. He lived for 40 days before choosing to forgo additional treatment after the transplant began to fail due to rejection.

Wednesday, January 31, 2024
Renowned Surgeon Dr. Bartley Griffith Named Vice Chair for Innovation in UM School of Medicine’s Department of Surgery
University of Maryland School of Medicine (UMSOM) Department of Surgery Chair Christine Lau, MD, MBA, along with UMSOM Dean Mark T. Gladwin, MD, announced today the appointment of Bartley P. Griffith, MD, as the Department of Surgery’s first Vice Chair for Innovation. In this role, Dr. Griffith will nurture a culture of innovation, entrepreneurship, and collaboration in the Department, and expand the integration of related sciences into surgical practice. The appointment is effective on February 1.
Tuesday, October 31, 2023
In Memoriam: Lawrence Faucette
It is with great sadness that we announce the passing of Lawrence Faucette, the 58-year-old patient with terminal heart disease who received the world’s second genetically-modified pig heart transplant. Mr. Faucette received the transplant on September 20 and lived for nearly six weeks following the surgery.

Friday, September 22, 2023
UM Medicine Faculty-Scientists and Clinicians Perform Second Historic Transplant of Pig Heart into Patient with End-Stage Cardiovascular Disease
A 58-year-old patient with terminal heart disease became the second patient in the world to receive a historic transplant of a genetically-modified pig heart on September 20. He is recovering and communicating with his loved ones. This is only the second time in the world that a genetically modified pig heart has been transplanted into a living patient. Both historic surgeries were performed by University of Maryland School of Medicine (UMSOM) faculty at the University of Maryland Medical Center (UMMC).
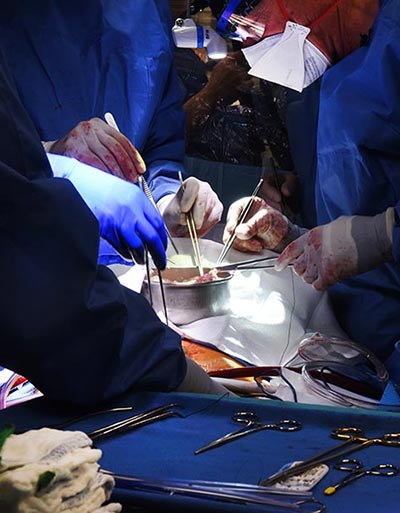
Friday, June 30, 2023
Lessons Learned from World’s First Successful Transplant of Genetically-Modified Pig Heart into Human Patient
A new study published today in the Lancet has revealed the most extensive analysis to date on what led to the eventual heart failure in the world's first successful transplant of a genetically-modified pig heart into a human patient. This groundbreaking procedure was conducted by University of Maryland School of Medicine (UMSOM) physician-scientists back in January 2022 and marked an important milestone for medical science.

Friday, December 16, 2022
UM School of Medicine Surgeon-Scientist Named One of Nature’s 10 People Who Helped Shape the Science Stories of 2022
The world-renowned journal Nature, named Muhammad Mohiuddin, MD, DSc, Program and Scientific Director of the Cardiac Xenotransplantation Program at the University of Maryland School of Medicine (UMSOM), on its annual list of 10 people who helped shaped science in 2022. His pivotal work over the past three decades transplanting genetically-modified pig hearts into non-human primates led to the historic xenotransplant of a pig heart into a human patient this past January. The surgery was led by Bartley Griffith, MD, the Thomas E. and Alice Marie Hales Distinguished Professor of Transplant Surgery and Clinical Director of the Cardiac Xenotransplantation Program, who was also recognized by Nature for his ground-breaking efforts to move the field of transplantation into a new era.
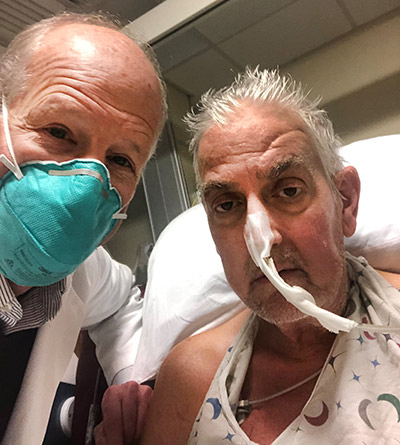
Wednesday, June 22, 2022
University of Maryland School of Medicine Faculty Scientists and Clinicians Publish Findings of World’s First Successful Transplant of Genetically Modified Pig Heart into Human Patient
Six months ago, University of Maryland School of Medicine surgeon-scientists successfully implanted a genetically modified pig heart into a 57 year-old patient with terminal heart disease in a first-of-its-kind surgery. It was considered an early success because the patient lived for two months with a strong functioning heart showing no obvious signs of rejection, according to a new paper published today in the New England Journal of Medicine.