March 20, 2018 | Joanne Morrison

Influenza Vaccine Study Will Test the Vaccine’s Safety and Ability to Generate an Immune Response
Vaccine experts at the University of Maryland School of Medicine (UMSOM) have begun multiple clinical trials of vaccines designed to protect against H7N9, an avian influenza virus that was first reported in humans in 2013 in China.
“This research will help us better understand immune responses to the vaccine,” said Wilbur Chen, MD, Associate Professor of Medicine and Chief of the Adult Clinical Studies section in UMSOM’s Center for Vaccine Development (CVD), who is leading one of the trials. “Pandemic preparedness is a priority. While the H7N9 virus is not circulating in the United States at this time, this important research will help us better understand how to protect individuals from the H7N9 influenza strain should it spread outside China.”
CVD has a Vaccine and Treatment Evaluation Unit (VTEU) funded by the National Institute of Allergy and Infectious Diseases (NIAID) (HHSN2720002I-FY.2017.B8C12.0080), an institute of the National Institutes of Health (NIH). Similar research is being conducted at other NIAID VTEU sites.
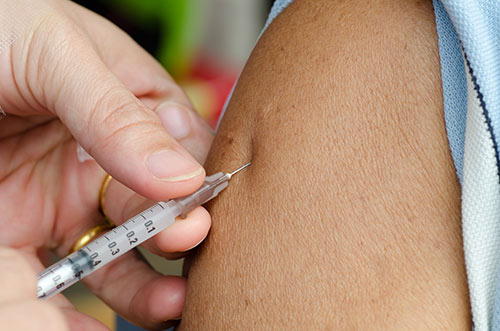 One trial at UMSOM involves healthy volunteers 19 years of age and older; it is the only trial evaluating the vaccine in elderly adults (age 65 years and older). This trial will evaluate different dosages of vaccine with or without an adjuvant, an ingredient used to stimulate better immune responses to a vaccine. The other trial will test the vaccine on adults 19 to 64 when given sequentially or simultaneously with seasonal influenza vaccine. In both these trials, the vaccine was developed by Sanofi Pasteur, based in Lyon, France. The vaccine – called 2017 H7N9 IIV– uses an inactivated form of the H7N9 influenza virus that was collected in 2017 to increase the likelihood that it will provide immunity against a newly evolved strain of the virus. The adjuvant, called AS03, was produced by GSK Biologicals, based in Rockville, MD, USA.
One trial at UMSOM involves healthy volunteers 19 years of age and older; it is the only trial evaluating the vaccine in elderly adults (age 65 years and older). This trial will evaluate different dosages of vaccine with or without an adjuvant, an ingredient used to stimulate better immune responses to a vaccine. The other trial will test the vaccine on adults 19 to 64 when given sequentially or simultaneously with seasonal influenza vaccine. In both these trials, the vaccine was developed by Sanofi Pasteur, based in Lyon, France. The vaccine – called 2017 H7N9 IIV– uses an inactivated form of the H7N9 influenza virus that was collected in 2017 to increase the likelihood that it will provide immunity against a newly evolved strain of the virus. The adjuvant, called AS03, was produced by GSK Biologicals, based in Rockville, MD, USA.
“Vaccine development and testing has been a key part of our research program here at the University of Maryland School of Medicine,” said UMSOM Dean E. Albert Reece, MD, PhD, MBA, Executive Vice President, Medical Affairs, University of Maryland and the John and Akiko K. Bowers Distinguished Professor and Dean, University of Maryland School of Medicine. “Influenza vaccine development is important, particularly as new strains arise each year and we face the the risk of potential pandemics.”
CVD researchers are recruiting volunteers for each of the trials. For more information call 410-706-6156 (8 a.m. - 4 p.m.) or visit http://www.medschool.umaryland.edu/CVD/Clinical-Trials-/.
About the University of Maryland School of Medicine
Commemorating its 210th Anniversary, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 43 academic departments, centers, institutes, and programs; and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Sciences, and a distinguished recipient of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically-based care for more than 1.2 million patients each year. The School has over 2,500 students, residents, and fellows, and nearly $450 million in extramural funding, with more than half of its academic departments ranked in the top 20 among all public medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total workforce of nearly 7,000 individuals. The combined School and Medical System (“University of Maryland Medicine”) has a total budget of $5 billion and an economic impact of nearly $15 billion on the state and local community. The School of Medicine faculty, which ranks as the 8th-highest public medical school in research productivity, is an innovator in translational medicine with 600 active patents and 24 start-up companies. The School works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu/.
About the Center for Vaccine Development
Since its inception in 1974, the CVD has worked to eliminate vaccine-preventable diseases. The CVD has created and tested vaccines against cholera, typhoid fever, paratyphoid fever, non-typhoidal salmonella disease, shigellosis (bacillary dysentery), Escherichia coli diarrhea, nosocomial pathogens, tularemia, influenza, and other infectious diseases. Learn more about the CVD.
Contact
Office of Public Affairs
655 West Baltimore Street
Bressler Research Building 14-002
Baltimore, Maryland 21201-1559
Contact Media Relations
(410) 706-5260
Related stories

Wednesday, October 08, 2025
New Vaccine Shows Promise Against Typhoid and Invasive Salmonella in First Human Trial
Researchers at the University of Maryland School of Medicine’s Center for Vaccine Development and Global Health (CVD) have completed a successful Phase 1 clinical trial of a novel vaccine designed to protect against both typhoid fever and invasive non-typhoidal Salmonella--two major causes of illness and death among children in sub-Saharan Africa.

Wednesday, March 12, 2025
Meningococcal Vaccine Found to be Safe and Effective for Infants in Sub-Saharan Africa
University of Maryland School of Medicine (UMSOM) researchers helped conduct an important new global health study that found a vaccine that protects against five strains of meningitis prevalent in sub-Saharan Africa is safe and effective for use in young children beginning at 9 months of age. This study provided evidence that formed the basis for the World Health Organization’s (WHO) decision last year to recommend the pentavalent Men5CV meningitis vaccine for infants ages 9 months and older.

Tuesday, April 30, 2024
Miriam K. Laufer Appointed Interim Director of the Center for Vaccine Development and Global Health
The University of Maryland School of Medicine (UMSOM) Dean, Mark T. Gladwin, MD, announced that Miriam K. Laufer, MD, Professor of Pediatrics, Medicine, and Epidemiology & Public Health, has been appointed as the Interim Head of UMSOM's Center for Vaccine Development and Global Health (CVD).

Tuesday, April 30, 2024
Miriam K. Laufer Appointed Interim Director of the Center for Vaccine Development and Global Health
University of Maryland School of Medicine (UMSOM) Dean, Mark T. Gladwin, MD, announced that Miriam K. Laufer, MD, Professor of Pediatrics, Medicine, and Epidemiology & Public Health, has been appointed as the Interim Head of UMSOM's Center for Vaccine Development and Global Health (CVD).

Thursday, March 28, 2024
NIH selects Dr. Kathleen Neuzil as Director of The Fogarty International Center
Kathleen M. Neuzil, MD, MPH, Director of the University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health, has been named the 13th director of the Fogarty International Center (FIC), which is part of the National Institutes of Health (NIH). Dr. Neuzil will be the first woman to hold the permanent directorship since the center’s founding in 1968 and will also hold the position of Associate Director for International Research at NIH.

Thursday, October 12, 2023
UM School of Medicine's Kirsten Lyke Elected as Member of Prestigious National Academy of Medicine
Kirsten E. Lyke, MD, Professor of Medicine and Physician-Scientist at the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM), was elected this week as a new member of the National Academy of Medicine (NAM). She was recognized for her pivotal research in emerging infections and human challenge models that have informed and shaped global vaccine and public health policy.

Monday, October 02, 2023
UM School of Medicine Researchers Present Interim Results on Meningococcal Vaccine for Infants and Young Children in Africa
University of Maryland School of Medicine (UMSOM) researchers, as part of the Infectious Diseases Clinical Research Consortium (IDCRC), provided an interim analysis showing that the pentavalent (NmCV-5) meningitis vaccine is safe for use in 9-month-old infants in the meningitis belt of sub-Saharan Africa. They presented their results to the World Health Organization’s (WHO) Strategic Advisory Group of Experts (SAGE) on Immunization on September 26.

Thursday, January 26, 2023
Small Study Shows Promise for Antimalarial Monoclonal Antibody to Prevent Malaria
A monoclonal antibody treatment was found to be safe, well tolerated, and effective in protecting against malaria in a small group of healthy volunteers who were exposed to malaria in a challenge study, according to new research published in by researchers at the University of Maryland School of Medicine (UMSOM).
Tuesday, August 09, 2022
New Study Confirms Typhoid Vaccine Safety, Immune Response in Children
A new study, published in The Lancet Global Health, finds typhoid conjugate vaccine, Typbar TCV®, provides immunity for up to 3 years in children as young as 9 months old in Malawi. The research – conducted by the Blantyre Malaria Project, Malawi-Liverpool-Wellcome Trust, and researchers at the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM) – found that the TCV vaccine is safe and well tolerated. Importantly, the vaccine can be given to 9-month-old infants at the same time as routine measles-rubella vaccinations without reducing the immune response to either vaccine.

Monday, August 08, 2022
New Study Finds Rapid Decline in Vaccine-Boosted Neutralizing Antibodies Against Omicron Subvariant BA.5
A study led in part by investigators at the University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health found that although COVID-19 booster vaccinations in adults elicit high levels of neutralizing antibodies against the Omicron variant of SARS-CoV-2, those antibody levels decrease substantially within three months. Kirsten E. Lyke, MD, Professor of Medicine at UMSOM and scientist at CVD, is Co-Chair and site Principal Investigator for the study, and Meagan Deming, MD, PhD, Assistant Professor of Medicine at the UMSOM, also a scientist at CVD, is Vice-Chair of the study, which is a collaboration between investigators at the UMSOM’s CVD and the Institute of Human Virology (IHV).
Thursday, March 24, 2022
UM School of Medicine Leads Research to Assess Meningococcal Vaccine for Infants and Young Children in Africa
Researchers at the University of Maryland School of Medicine (UMSOM)’s Center for Vaccine Development & Global Health (CVD) are leading a study to evaluate the use of a pentavalent – or five in one – meningococcal conjugate vaccine (NmCV-5) among infants and young children in the meningitis belt of sub-Saharan Africa. This is the final and pivotal study for World Health Organization (WHO) prequalification of this vaccine, which is the last stage to make the vaccine available for low- and middle-income countries.

Thursday, September 16, 2021
First Efficacy Results from Africa find Typhoid Vaccine to offer 84 Percent Protection against Typhoid Fever
A new study, published in the New England Journal of Medicine, finds a single dose of typhoid conjugate vaccine (TCV) – the only typhoid vaccine licensed for children as young as 6 months – is safe and 84 percent effective in protecting against typhoid in Blantyre, Malawi. These are the first efficacy results from Africa and part of a five-year, multi-country project to accelerate introduction of TCV.

Wednesday, January 27, 2021
Dr. Wilbur Chen, Nationally-Recognized Vaccine Researcher, Selected for Federal Committee that Guides Immunization Policies
Wilbur H. Chen, MD, MS, FIDSA, FACP, Professor of Medicine at the University of Maryland School of Medicine (UMSOM), has been named a new voting member of the federal government’s Advisory Committee on Immunization Practices (ACIP), the prestigious board of experts that makes recommendations on the safe use of vaccines for Americans. The U.S. Department of Health and Human Services selected Dr. Chen for the 15-member advisory committee based on his expertise and national leadership in vaccinology, infectious diseases, public health, and preventive medicine. He will remain in his current role at UMSOM while he serves in his four-year term, which began last month.

Tuesday, February 25, 2020
World Health Organization Names UMSOM Faculty Member as COVID-19 Advisor
Samba Sow, MD, MSc, FASTMH, Director General of the Center for Vaccine Development in Mali (CVD-Mali), and Adjunct Professor of Medicine at the University of Maryland School of Medicine (UMSOM), was appointed by the World Health Organization (WHO) to serve as a special envoy on issues related to coronavirus COVID-19.

Tuesday, December 10, 2019
UMSOM Researchers to Test Vaccine Designed to Protect Against Serious Illness from Contaminated Food and Water
Each year, millions of people contract serious diarrheal illnesses typically from contaminated food and water. Among the biggest causes of diarrheal diseases are the bacteria Shigella and enterotoxigenic Escherichia coli (ETEC), and researchers at the University of Maryland School of Medicine are testing a vaccine designed to offer protection against these serious pathogens.

Friday, February 23, 2018
University of Maryland School of Medicine Vaccine Expert Highlights Need for Vaccination Among Older Adults During Capitol Hill Briefing
Today at a briefing on Capitol Hill, Wilbur Chen, MD, MS, Associate Professor of Medicine at the University of Maryland School of Medicine, warned that the U.S. population of adults 65 and older is expected to rise significantly over the next few decades, making vaccinations against diseases like influenza, pneumonia and shingles for this population very critical.

Tuesday, November 21, 2017
UM SOM Scientist Elected as Fellow of American Society of Tropical Medicine and Hygiene
Robert Edelman, MD, a Clinical Professor of Medicine and Pediatrics at the University of Maryland School of Medicine (UM SOM), has been elected as a Fellow by the Board of Directors of the American Society of Tropical Medicine and Hygiene (ASTMH). This honor was awarded for his sustained professional excellence in tropical medicine, hygiene, global health, and related disciplines.
Friday, January 13, 2017
Scientists Identify Vaccination as the Most Cost-Effective Strategy to Sharply Reduce Rabies Deaths in India
Every year in India, 20,000 people are estimated to die from rabies. Most of the victims are children. Nearly all of the deaths occur after victims are bitten by rabid dogs. For years, experts have debated the best strategy to reduce this burden.

Friday, December 23, 2016
Trial Results Confirm Ebola Vaccine Provides High Protection Against Disease
An experimental Ebola vaccine was highly protective against the deadly virus in a major trial in Guinea, according to a new study that included researchers from the University of Maryland School of Medicine (UM SOM).

Friday, June 10, 2016
FDA Approves Vaccine for Cholera Invented and Developed at University of Maryland School of Medicine
In a milestone that was years in the making, a vaccine to prevent cholera, invented and developed by researchers at the University of Maryland School of Medicine’s Center for Vaccine Development, was approved today by the U.S. Food and Drug Administration (FDA).

Monday, January 11, 2016
UM SOM to Collaborate with Industry to Develop a Vaccine to Prevent Common, Deadly Infection
The Center for Vaccine Development (CVD) at the University of Maryland School of Medicine (UM SOM) will participate in a partnership with industry to develop a vaccine to prevent a group of deadly bacterial infections that occur commonly among hospital patients
