Flow cytometry is a technique used to detect and measure physical and biochemical features of cells. Historically, this has been a powerful tool for immunologists, allowing for characterization of distinct leukocyte populations using a combination of surface and intracellular antigen labeling. In recent years, this approach has proven useful in understanding the role of the immune response to brain injury. Advances in this area have expanded its utility to neuroscience applications, allowing for measurement in other neural cell types, such as neuronal apoptosis, reactive oxygen species generation, and calcium signaling. Clinical studies have demonstrated the prognostic value of blood leukocyte enumeration in TBI patients1,2, which have now been replicated in experimental animal models3. Flow cytometry provides an important window into the body’s immune response to brain injury and has proven a valuable tool for developing new therapeutics to treat patients.
Our laboratory has had a long-running interest in neuroinflammation in relation to CNS trauma. The research program here is dedicated to unraveling the mechanisms by which inflammation drives the progression of chronic neurodegeneration after TBI. By implementing a flow cytometry approach to study the inflammatory dynamics in TBI, our lab has made several novel discoveries. These include elucidating the impact of age4 and sex5 on the neuroinflammatory response to TBI, including changes in systemic immunity3. This work, led by Dr. Rodney Ritzel, has demonstrated body-wide changes in immune cell number and function after brain injury, which varies across tissue and time. These studies have identified new cellular targets for therapy and highlight the utility of cutting-edge multi-functional ex-vivo flow cytometry applications for neurological disease. The latter innovation allows us to assess multiple functions simultaneously in multiple cell types, which provides higher resolution of how cellular functions work in concert following brain injury. These functions include cytokine production, mitochondrial activity, protein phosphorylation, enzymatic activity, cell proliferation, and cell death. More sophisticated techniques, like flow cytometry, aid our understanding of brain inflammation and enable our laboratory to stay at the cutting edge of TBI research.
Recently, our laboratory has shown that TBI increases the severity of lung6 and gut7 bacterial infection, exacerbating secondary injury in the brain. These findings suggest a bi-directional relationship between the nervous system and the immune system after head injury.
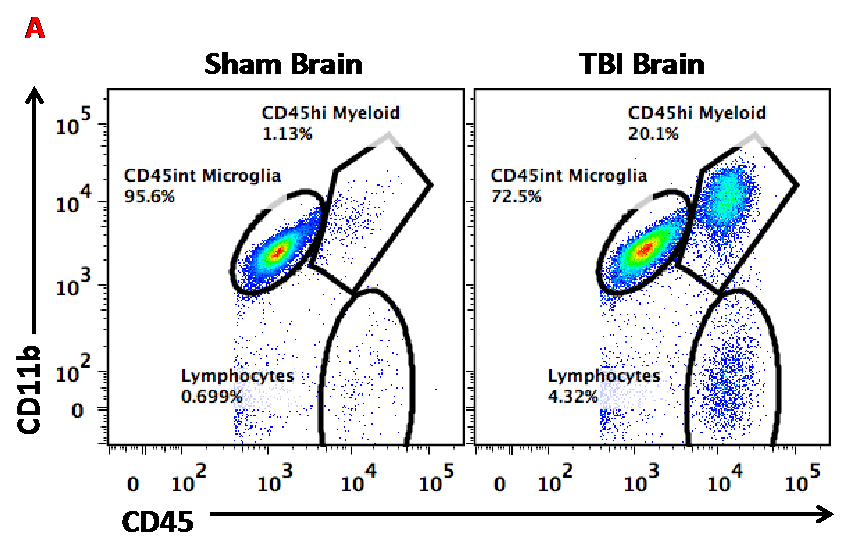
Figure A. This dot plot illustrates how flow cytometry is useful for discriminating between resident microglia and brain-infiltrating macrophages after TBI.
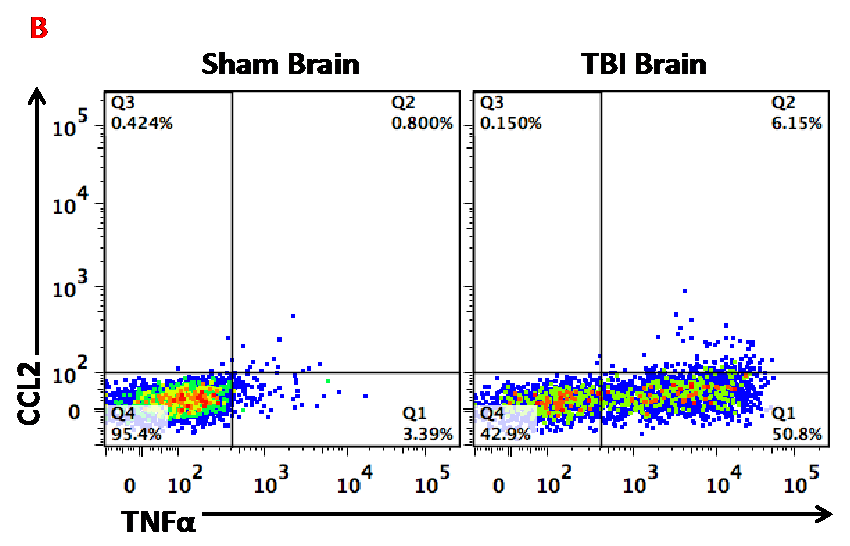
Figure B. This dot plot shows the utility of flow cytometry for measuring microglia activation. In this case, cytokine production of TNF and CCL2 was measured in microglia from sham and TBI mice.
References Cited
1. Yu P, Tian Q, Wen X, Zhang Z, Jiang R. Analysis of long-term prognosis and prognostic predictors in severe brain injury patients undergoing decompressive craniectomy and standard care. Journal of Craniofacial Surgery. 2015 Oct 1;26(7):e635-41.
2. Rhind SG, Crnko NT, Baker AJ, Morrison LJ, Shek PN, Scarpelini S, Rizoli SB. Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. Journal of neuroinflammation. 2010 Dec;7(1):5.
3. Ritzel RM, Doran SJ, Barrett JP, Henry RJ, Ma EL, Faden AI, Loane DJ. Chronic alterations in systemic immune function after traumatic brain injury. Journal of neurotrauma. 2018 Jul 1;35(13):1419-36.
4. Ritzel RM, Doran SJ, Glaser EP, Meadows VE, Faden AI, Stoica BA, Loane DJ. Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes following acute traumatic brain injury in mice. Neurobiology of Aging. 2019 Feb 20.
5. Doran SJ, Ritzel RM, Glaser EP, Henry RJ, Faden AI, Loane DJ. Sex differences in acute neuroinflammation after experimental traumatic brain injury are mediated by infiltrating myeloid cells. Journal of neurotrauma. 2018 Nov 16.
6. Doran SJ, Henry RJ, Shirey KA, Barrett JP, Ritzel RM, Lai W, Blanco JC, Faden AI, Vogel SN, Loane DJ. Early or late bacterial lung infection increases mortality after traumatic brain injury in male mice and chronically impairs monocyte innate immune function. Read Online: Critical Care Medicine| Society of Critical Care Medicine. 2020 May 1;48(5):e418-28.
7. Ma EL, Smith AD, Desai N, Cheung L, Hanscom M, Stoica BA, Loane DJ, Shea-Donohue T, Faden AI. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain, behavior, and immunity. 2017 Nov 1;66:56-69.
