November 04, 2022 | Vanessa McMains
Drug Lessens Harm and May Serve as a Potential COVID-19 Therapy
Researchers at the University of Maryland School of Medicine’s (UMSOM) Center for Precision Disease Modeling identified how a specific protein in SARS-CoV-2, the virus responsible for COVID-19, damages heart tissue. They then used a drug to reverse the toxic effects of that protein on the heart.
 Their findings, based on research with fruit flies and mouse heart cells, were published on Sept. 30, 2022, in Communications Biology, a Nature journal.
Their findings, based on research with fruit flies and mouse heart cells, were published on Sept. 30, 2022, in Communications Biology, a Nature journal.
People infected with COVID-19 are at a significantly higher risk for developing inflammation of the heart muscle, abnormal heart rhythms, blood clots, stroke, heart attacks, and heart failure for at least a year after infection, compared to those who have not been infected with the virus. Although scientists rapidly developed vaccines and medications to lessen the severity of COVID-19 disease, these therapies do not protect the heart or other organs from the damage that can be done by even a mild infection.
“To treat patients in the long run, we must first understand the mechanism behind what is causing the disease. Our research shows that individual SARS-CoV-2 proteins can each do major damage to specific tissues in the body — similar to what has been found for other viruses like HIV and Zika,” said senior author Zhe Han, PhD, Professor of Medicine and Director of the Center for Precision Disease Modeling at UMSOM. “By identifying these processes of injury in each tissue, we can test drugs to see whether any can reverse this damage; those drugs that show promise can then be further tested in clinical research studies.”
Last year, Dr. Han and his research team identified the most toxic SARS-CoV-2 proteins in studies using fruit flies and human cells. They found a promising drug selinexor reduced the toxicity of one of these proteins, but not the other one, known as Nsp6.
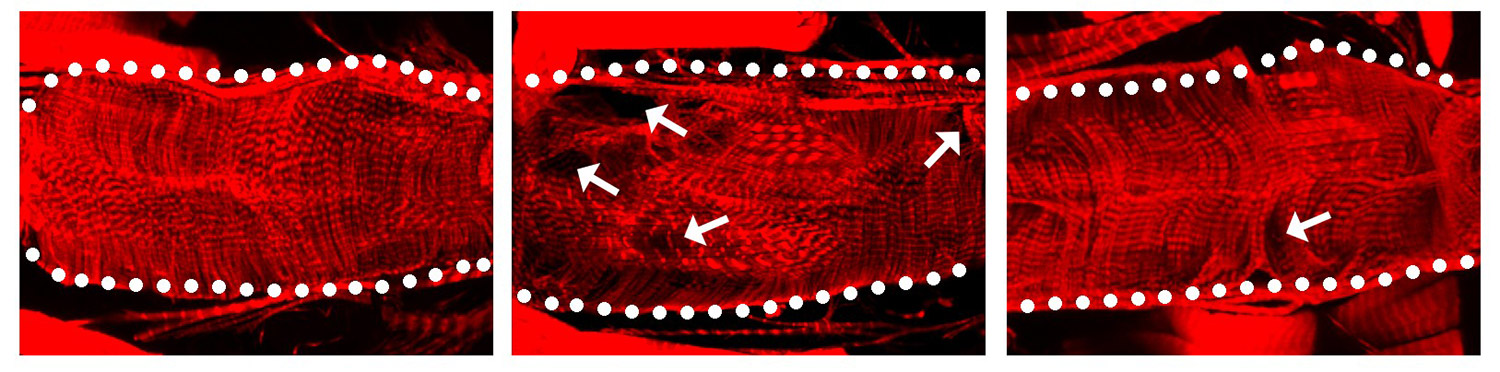
The image above shows: When the SARS-CoV-2 protein Nsp6 is made in a fruit fly heart (center), the heart has structural defects compared to a normal heart without the viral protein (left). When fruit fly hearts with the viral Nsp6 protein are given the metabolism changing drug 2DG (right), the hearts begin to look more normal than the ones with the viral protein that do not have the drug.
In their latest study, they found that Nsp6 turned out to be the most toxic SARS-CoV-2 protein in the fly heart. Next, they found that the Nsp6 protein hijacked the fruit fly’s cells in its heart to turn on the glycolysis process, which enables cells to burn the sugar glucose for energy. Typically, heart cells use fatty acids as an energy source, but switch over to sugar metabolism during heart failure as these cells to try to repair the damaged tissue. The researchers also found the Nsp6 protein did added damage by disrupting the cell’s powerhouse, called the mitochondria, which produces energy from sugar metabolism.
The team then blocked sugar metabolism in fruit flies and mouse heart cells using the drug 2-deoxy-D-glucose (2DG). They found that the drug reduced the heart and mitochondria damage caused by the Nsp6 viral protein.
 “We know that some viruses hijack the infected animal’s cell machinery to change its metabolism to steal the cell’s energy source, so we suspect SARS-CoV-2 does something similar. The viruses can also use the byproducts of sugar metabolism as building blocks to make more viruses,” said Dr. Han. “So, we predict this drug that changes the metabolism in the heart back to what it was before infection would be bad for the virus, by both cutting off its energy supply and eliminating the pieces it needs to replicate.”
“We know that some viruses hijack the infected animal’s cell machinery to change its metabolism to steal the cell’s energy source, so we suspect SARS-CoV-2 does something similar. The viruses can also use the byproducts of sugar metabolism as building blocks to make more viruses,” said Dr. Han. “So, we predict this drug that changes the metabolism in the heart back to what it was before infection would be bad for the virus, by both cutting off its energy supply and eliminating the pieces it needs to replicate.”
The researchers said that fortunately 2DG is inexpensive and is used regularly in laboratory research. Although 2DG has not been approved by the U.S. Food and Drug Administration to treat disease, the drug is currently in clinical trials for treatment of COVID-19 in India.
“Too many Americans who have recovered from COVID wind up with dangerous heart conditions weeks or months later, and we need to learn the fundamental reasons for why this is happening,” said Mark T. Gladwin, MD, Vice President for Medical Affairs at University of Maryland, Baltimore and the John Z. and Akiko K. Bowers Distinguished Professor and Dean, UMSOM. “With this research elucidating the pathways of the Nsp6 protein, we can refine the treatments we target for future research with the ultimate aim of reversing further heart damage in these patients.”
This study was funded by the University of Maryland, Baltimore Institute for Clinical and Translational Research COVID-19 Accelerated Translational Incubator Pilot (ATIP) grant.
For More Information
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world — with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.3 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic, and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
Contact
Vanessa McMains
Director, Media & Public Affairs
University of Maryland School of Medicine
Institute of Human Virology
vmcmains@ihv.umaryland.edu
Cell: 443-875-6099
Related stories

Friday, April 03, 2020
UM School of Medicine Researchers Identify Mechanism to Explain Role of Certain Gene Mutations in Kidney Disease
Researchers from the Center for Precision Disease Modeling at the University of Maryland School of Medicine (UMSOM) have uncovered a mechanism that appears to explain how certain genetic mutations give rise to a rare genetic kidney disorder called nephrotic syndrome. Using a drosophila (fruit fly) model, they found mutations in genes that code for certain proteins lead to a disruption of the recycling of the cell membrane. This disruption leads to an abnormal kidney cell structure and function, according to the study published this week in the Journal of the American Society of Nephrology.

Wednesday, September 25, 2019
UM School of Medicine Scientist Receives NIH Award to Study Heart Disease Related to Down Syndrome
The National Institutes of Health announced today that Zhe Han, PhD, Associate Professor in the Department of Medicine at the University of Maryland School of Medicine (UMSOM), will be awarded a supplemental funding of $772,500 to the ongoing $1.75 million NIH-funded project in Dr. Han’s lab for studying genes involved in Congenital Heart Disease. This supplemental fund will support the research to identify the genetic causes of Congenital Heart Disease that occur in half of all patients with Down syndrome. The goal is to identify genes associated with Down syndrome congenital heart disease and to study the molecular mechanism underlying the cardiac defects. This can potentially lead to better treatments for these heart abnormalities which are a major cause of mortality in those with Down syndrome.