June 21, 2021 | Vanessa McMains
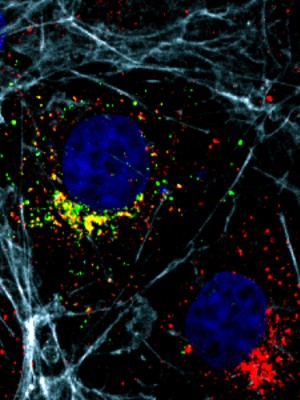
Transport system of essential materials in brain cells disrupted in certain genetic developmental disorders.
University of Maryland School of Medicine (UMSOM) researchers have identified a new gene that may be linked to certain neurodevelopmental disorders and intellectual disabilities. The researchers believe that finding genes involved in certain types of developmental disorders provide an important first step in determining the cause of these disorders and ultimately in developing potential therapies for treating them. A paper documenting their discovery was recently published in the American Journal of Human Genetics.
About 3 percent of the world’s population has an intellectual disability. Up to half of the cases are due to genetics, however, because many thousands of genes contribute to brain development, it has been difficult to identify the specific cause for each patient.
Once researchers identified the gene, they worked with collaborators to give clinical diagnoses to 10 other families around the world, who had relatives with this condition. The researchers also used zebrafish to show the gene’s role in development and survival, demonstrating its importance in helping the brain’s neurons function properly.
“Our goal is to find as many of these genes required for brain function and take this knowledge back to patients and families to provide a clinically relevant genetic diagnosis,” said Saima Riazuddin, PhD, MPH, MBA, Professor of Otorhinolaryngology-Head & Neck Surgery and Biochemistry & Molecular Biology at UMSOM.
Dr. Riazuddin and her team collaborate regularly with several scientists in Pakistan studying a group of 350 families who are geographically isolated, which as a result has led to inbreeding and resulted in genetic disorders such as neurodevelopmental disorder and intellectual disability.
_300W.jpg)
The research team focused on one particular family with two brothers and an uncle with symptoms of intellectual disability, delayed speech and other developmental milestones and epilepsy. Other members of the family who had similar symptoms had since passed in childhood or early adulthood. Dr. Riazuddin and her team identified the gene AP1G1 as the culprit.
Then through collaboration with 27 other institutions, her team was able to identify ten other families with variations in the same gene that led to growth retardation and intellectual disability. These families lived in Italy, Germany, the Netherlands, Poland, and the United States.
To determine the gene’s role in development, the researchers engineered zebrafish without Ap1g1. These zebrafish embryos all began to die off by the fourth day. When the researchers added back mutated versions of the genes, like those found in the families with neurodevelopment disorder and intellectual disability, they observed a spectrum of symptoms with some zebrafish embryos dying off, some with major structural defects, and others with only minor tail deformities.
The gene AP1G1 contains the blueprints to make the protein Adaptor Protein 1 gamma 1 (AP1γ1). This protein is one of five pieces that make up the Adaptor Protein Complex, which builds transport vesicles to move materials around cells.

“Think of these transport vesicles as little vehicles like trucks that have to load, transport, and unload their cargo around the cells (e.g. neurons) to provide the necessary supplies for the cell to function,” said Dr. Riazuddin.
Dr. Riazuddin’s team made normal and mutant versions of AP1G1 that they put in mammalian cells with cargo molecules labeled in red. The cells with the mutant versions of AP1G1 had vesicles that were delayed in delivering their cargo or did not make their deliveries at all.
“Improving clinical diagnosis of these developmental disorders may eventually provide new targets for therapies, in order to one day be able to treat these conditions allowing more people to live independently,” said E. Albert Reece, MD, PhD, MBA, Executive Vice President for Medical Affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor and Dean, University of Maryland School of Medicine.
This studied was funded by the National Institute of Neurological Disorders and Stroke (R01NS107428), the EU FP7 Large-Scale Integrating Project Genetic and Epigenetic Networks in Cognitive Dysfunction (241995), Higher Education Commission of Pakistan (NRPU project 10700), and a Fondazione del Monte grant (ID ROL: FDM/4021).
The researchers have no conflicts of interest to declare.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.2 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
Contact
Vanessa McMains
Director, Media & Public Affairs
Institute of Human Virology
University of Maryland School of Medicine
vmcmains@ihv.umaryland.edu
Cell: 443-875-6099
Related stories

Tuesday, November 21, 2017
University of Maryland School of Medicine Researchers Set Standards for What Diseases Should Be Called
Researchers at the University of Maryland School of Medicine (UM SOM) are searching through medical journals, databases and other authoritative sources to establish standard terms for diseases as a way to help future researchers better understand a wide range of diseases like cancer. The work involves a time-intensive manual curation process, examining the breadth of information, naming systems and levels of evidence in order to integrate information and bridge data across resources.
Wednesday, May 31, 2017
When It Comes to Health, Does Zip Code Matter More than Genetic Code?
A range of powerful evidence shows unequivocally that where you live, as well as your social circumstances, play a huge role in your health. This was the message delivered by Anthony B. Iton, MD, JD, MPH, Senior Vice President of Healthy Communities at the California Endowment, in a lecture April 18 at the University of Maryland School of Medicine (UM SOM).
