June 22, 2023
Open Label Study Aims to Ensure Safety, Immune Response in This Age Group
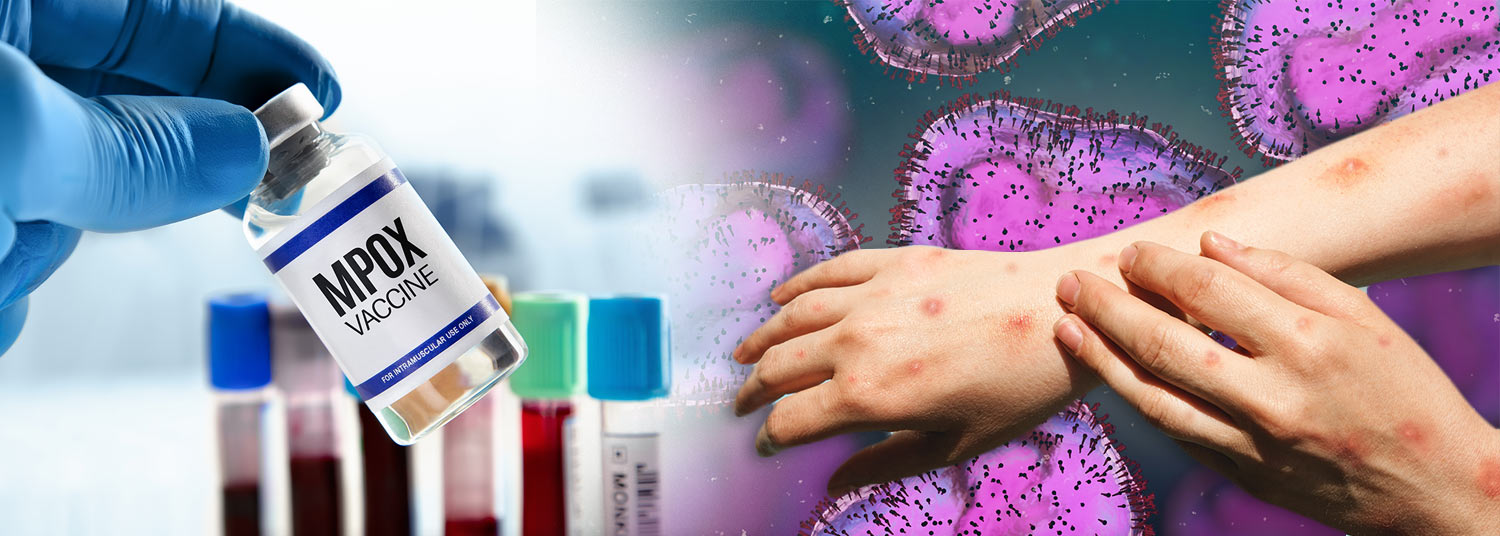
A 2022 outbreak of mpox (formerly monkeypox) sickened more than 30,000 people and caused 38 deaths in the United States. It highlighted the lack of an approved vaccine for those under 18 years old. To address this pressing need, faculty-scientists at the University of Maryland School of Medicine (UMSOM) recently launched a phase 2 safety trial to test a vaccine in adolescents ages 12 to 17.
The vaccine, JYNNEOS (MVA-BN), was approved by the U.S. Food and Drug Administration (FDA) in 2019 for use with adults at risk for mpox. In 2022, this vaccine was authorized on an emergency use basis for use with patients under 18 years of age during the outbreak. The latest stage of the trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
 The Phase 2 clinical trial will investigate whether the vaccine is safe and triggers an immune response in adolescents ages 12 to 17 that is comparable in adults ages 18 to 50 years. "While the risk of children and adolescents in the U.S. getting infected with mpox is currently low, it remains important to ensure that currently available vaccines are safe and lead to good immune responses in teenagers," said James Campbell, MD, MS, Professor of Pediatrics at UM SOM and principal investigator of the new mpox study's UM SOM site. "Children and adolescents can become infected with mpox through various modes of transmission, including close contact with people or animals infected with mpox, contact with contaminated materials, and sexual contact. This includes cuddling, caregiving, bed-sharing, and contact with body fluids and respiratory secretions of patients with mpox."
The Phase 2 clinical trial will investigate whether the vaccine is safe and triggers an immune response in adolescents ages 12 to 17 that is comparable in adults ages 18 to 50 years. "While the risk of children and adolescents in the U.S. getting infected with mpox is currently low, it remains important to ensure that currently available vaccines are safe and lead to good immune responses in teenagers," said James Campbell, MD, MS, Professor of Pediatrics at UM SOM and principal investigator of the new mpox study's UM SOM site. "Children and adolescents can become infected with mpox through various modes of transmission, including close contact with people or animals infected with mpox, contact with contaminated materials, and sexual contact. This includes cuddling, caregiving, bed-sharing, and contact with body fluids and respiratory secretions of patients with mpox."
About 315 healthy adolescents will be enrolled in this study. They will receive the vaccine subcutaneously in the upper arm on day 1 and day 29, which is the dosage and timing used for adults. Participants will receive blood tests to check for immune responses. About 135 healthy adults will also receive the standard, licensed regimen of the vaccine also subcutaneously in the upper arm on day 1 and day 29. For analysis, information about this group of adults will be combined with the 76 healthy adults who received the standard regimen in a previous phase 1 study. The study is not placebo controlled, so all participants will receive a vaccine injected subcutaneously, just under the skin.
The purpose of this phase of the study is to gather additional data on the vaccine's safety and immune response in teens. The vaccine used in this study is licensed for adults for protection against mpox, but not for those under age 18. The vaccine is currently available for use after known exposure for children and adolescents under age 18 who are considered at high risk for mpox infection under an Emergency Use Authorization (EUA) issued by the U.S. Food and Drug Administration in August 2022. As of October 2022, at least one dose of this vaccine was given to 951 children aged 18 and younger under this EUA.
 "We are excited to study the immune response of JYNNEOS in children and adolescents for the first time;" said Elizabeth Hammershaimb, MD, Instructor of Pediatrics at UMSOM and also an investigator at UMSOM's mpox study site. "However, we are also reassured by the fact that the virus used in this vaccine has been extensively studied in children as young as S months old for other diseases, such as measles and Ebola, with no serious safety concerns reported.''
"We are excited to study the immune response of JYNNEOS in children and adolescents for the first time;" said Elizabeth Hammershaimb, MD, Instructor of Pediatrics at UMSOM and also an investigator at UMSOM's mpox study site. "However, we are also reassured by the fact that the virus used in this vaccine has been extensively studied in children as young as S months old for other diseases, such as measles and Ebola, with no serious safety concerns reported.''
JYNNEOS was administered to a few young children in the United Kingdom in 2018-2019, including infants, following exposures to mpox, with no known adverse events. It was also given to children in the U.S. during the recent mpox outbreak without any reported serious safety concerns.
UMSOM's participation is funded through a contract with Frederick National Laboratory for Cancer Research, operated by Leidos Biomedical Research in Frederick, Maryland, which provides scientific support to NIH.
For more information about the trial, visit ClinicalTrials.gov. The study's Clinical Trials Identifier is NCT05740982.
About the Center for Vaccine Development and Global Health at the University of Maryland School of Medicine
For over 40 years, researchers in the Center for Vaccine Development and Global Health (CVD). have worked domestically and internationally to develop, test, and deploy vaccines to aid the world's underserved populations. CVD is an academic enterprise engaged in the full range of infectious disease intervention, from basic laboratory research through vaccine development, pre-clinical and clinical evaluation, large-scale pre-licensure field studies, and post-licensure assessments. CVD has created and tested vaccines against cholera, typhoid fever, paratyphoid fever, non-typhoidal Salmonella disease, shigellosis (bacillary dysentery), Escherichia coli diarrhea, nosocomial pathogens, tularemia, influenza, coronaviruses, malaria, and other infectious diseases. CVD's research covers the broader goal of improving global health by conducting innovative, leading research in Baltimore and around the world. Our researchers are developing new and improved ways to diagnose, prevent, treat, control, and eliminate diseases of global impact, including COVID-19. In addition, CVD's work focuses on the ever-growing challenge of antimicrobial resistance.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.2 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System ("University of Maryland Medicine") has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu.
Contact
Office of Public Affairs
655 West Baltimore Street
Bressler Research Building 14-002
Baltimore, Maryland 21201-1559
Contact Media Relations
(410) 706-5260
Related stories
Wednesday, July 17, 2019
Vaccines Tested in UM School of Medicine’s Center for Vaccine Development Protect Children Around the World
For 30 years, the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM) has collaborated with the Pediatric Center of Frederick to test vaccines used in pediatric care.