October 13, 2022 | Deborah Kotz
Research Provides Reassurance to Orthopedic Surgeons and Helps Improve Practice of Evidence-Based Medicine
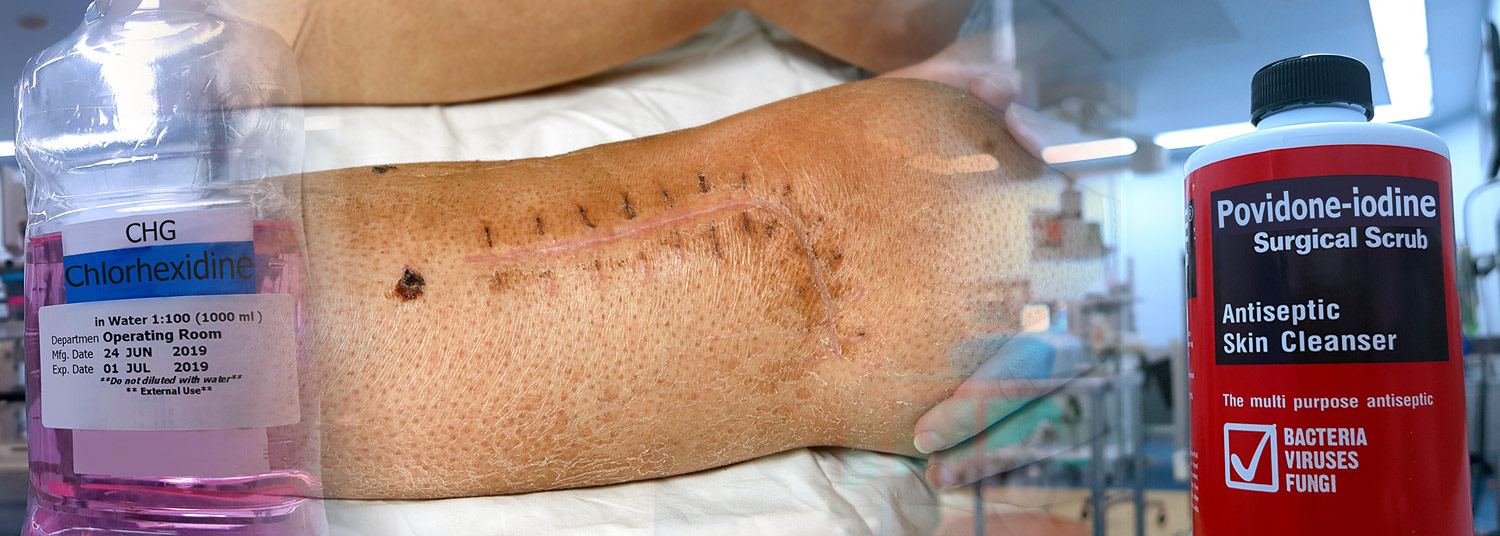
 A randomized clinical trial involving more than 1,600 patients with open fractures found that two antiseptic solutions routinely used by surgeons prior to fracture surgery are equally effective for preventing post-surgical infections. Based on the results of this trial, the study leaders from the University of Maryland School of Medicine (UMSOM) and McMaster University concluded that orthopedic surgeons could select either of the two solutions -- aqueous 10% povidone-iodine or aqueous 4% chlorhexidine gluconate – for infection prevention.
A randomized clinical trial involving more than 1,600 patients with open fractures found that two antiseptic solutions routinely used by surgeons prior to fracture surgery are equally effective for preventing post-surgical infections. Based on the results of this trial, the study leaders from the University of Maryland School of Medicine (UMSOM) and McMaster University concluded that orthopedic surgeons could select either of the two solutions -- aqueous 10% povidone-iodine or aqueous 4% chlorhexidine gluconate – for infection prevention.
The study, called Aqueous-PREP, was published in The Lancet and presented yesterday at the Orthopaedic Trauma Association meeting in Tampa, FL. Study participants were enrolled from a total of 14 hospitals, including the University of Maryland Medical Center (UMMC), as well as other facilities in the U.S., Canada, and Spain.
“Many surgeons prefer to use povidone-iodine skin preparation for open fractures because of its wide availability as an effective aqueous solution,” said study co-principal investigator Gerard Slobogean, MD, MPH, Associate Professor of Orthopedics at UMSOM and an orthopedic trauma surgeon at the R Adams Cowley Shock Trauma Center at UMMC. “Our findings should reassure surgeons that either antiseptic solution is fine to use, and they can base their decision on product availability, cost, or patient contraindications,” Dr. Slobogean added.
To conduct the trial, the researchers recruited patients with open fractures that required surgery at the 14 participating hospitals. Each hospital was randomly assigned to use one of the antiseptic formulations at the beginning of the three-year trial; then, each hospital alternated between the solutions every two months. This unique study design, known as a multiple period cluster randomized crossover trial, ensured that all the hospitals used both formulations equally and controlled for variations among hospitals that could have also affected post-surgical infection rates.
“Open fractures are often devastating injuries that can be challenging to study due to the severity of the injuries and their need for urgent surgery. Patient engagement and our novel study design were key to overcoming many of the logistical challenges,” said Sheila Sprague, PhD, MSc, co-principal investigator of the trial and an Associate Professor and Research Director at McMaster University.
 Jeff Wells, trial executive committee member and patient partner, agrees. “As a patient involved in the trial, it was my job to ensure that the voice of trauma patients was heard in the design, implementation, and dissemination of the trial. However, many months into the project, I learned that involving patients in study leadership was uncommon,” Wells said.
Jeff Wells, trial executive committee member and patient partner, agrees. “As a patient involved in the trial, it was my job to ensure that the voice of trauma patients was heard in the design, implementation, and dissemination of the trial. However, many months into the project, I learned that involving patients in study leadership was uncommon,” Wells said.
Added Andrew Pollak, MD, the James Lawrence Kernan Professor and Chair of the Department of Orthopedics at UMSOM and Senior Vice President and Chief Clinical Officer for the 11-hospital University of Maryland Medical System (UMMS), “This is a landmark study with important clinical implications. The findings are not only relevant to the management of open fractures but might also apply to the surgical treatment of other traumatic wounds.”
Aqueous-PREP is one of two trials being led by Dr. Slobogean and colleagues that are aimed at closing gaps in the medical literature on the most effective infection-control techniques in orthopedic surgery. The study designs follow a master protocol called PREP-IT (Program of Randomized Trials to Evaluate Pre-operative Antiseptic Skin Solutions in Orthopaedic Trauma) to test infection prevention techniques in trials that will provide crucial evidence to help guide surgical practices. The second study called PREPARE will evaluate the use of two alcohol-based antiseptic skin preparations in surgical patients with both open and closed lower extremity fractures.
 “Extremity fractures and the challenging surgical site infections that result from them pose a significant healthcare burden on our nation,” said Mark T. Gladwin, MD, Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor and Dean, University of Maryland School of Medicine. “The master protocol used in this study provides efficiencies in study design and data collection and could serve as an important model for future comparative effectiveness studies in the surgical field.”
“Extremity fractures and the challenging surgical site infections that result from them pose a significant healthcare burden on our nation,” said Mark T. Gladwin, MD, Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor and Dean, University of Maryland School of Medicine. “The master protocol used in this study provides efficiencies in study design and data collection and could serve as an important model for future comparative effectiveness studies in the surgical field.”
This research was supported by the PREP-IT Investigators, which includes a network of over 200 physicians, allied health care providers, trauma patients, and clinical researchers. This trial was funded by the US Department of Defense (W81XWH-17-1-070), the Canadian Institutes of Health Research (Foundation Grant), McMaster University Surgical Associates, and the PSI Foundation.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.3 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic, and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
Contact
Deborah Kotz
Senior Director of Media Relations
Office of Public Affairs & Communications
University of Maryland School of Medicine
Email: DKotz@som.umaryland.edu
o: 410-706-4255
c: 410-804-0054
t: @debkotz2
Related stories

Wednesday, January 31, 2024
Large Multicenter Clinical Trial Finds that Antiseptic Containing Iodine Reduces Surgical-Site Infections in Patients with Extremity Fractures
A large multicenter clinical trial co-led by University of Maryland School of Medicine researchers found that an antiseptic containing iodine resulted in about one-quarter fewer post-surgical infections in patients with limb fractures compared to another frequently used skin antiseptic. The results of the study of nearly 8,500 patients across the United States and Canada were published today in the New England Journal of Medicine.

Tuesday, November 21, 2017
University of Maryland School of Medicine Scientists Set to Begin Studies of Infection Prevention Related to Fracture Treatment
University of Maryland School of Medicine (UM SOM) researchers have been awarded more than $14 million to carry out two research projects to investigate the best way to prevent infections that can occur during the surgical treatment of fractures.