Academic Title:
Associate Professor
Primary Appointment:
Anesthesiology
Secondary Appointment(s):
Neurobiology
Additional Title:
Shock,Trauma and Anesthesiology Research (STAR) Center; Center for Stem Cell Biology and Regenerative Medicine; Center for Biomolecular Therapeutics
Location:
MSTF, 6-34E
Phone (Primary):
(410) 706-5187
Fax:
(410) 706-1639
Education and Training
- BA: Indiana University, Bloomington, IN
- PhD: Massachusetts Institute of Technology, Cambridge, MA
- Postdoctoral training: Harvard Medical School, Boston, MA
Biosketch
Lab website: https://www.lipinski-lab.org/
Additional Affiliations at UMB
- Member, Shock, Trauma and Anesthesiology Research (STAR) Center
- Member & Neural Stem Cell Interest Group Leader, Center for Stem Cell Biology and Regenerative Medicine
- Member, Center for Biomolecular Therapeutics
Research/Clinical Keywords
Autophagy, Lysosomes, Neuroinflammation, Lipid Metabolism, Traumatic Brain Injury, Spinal Cord Injury, Neurodegenerative Disease, Brain Aging
Highlighted Publications
Selected Recent Publications
Choi HMC, Li Y, Suraj D, Hsia RC, Wu J# and Lipinski MM#. Autophagy protein ULK1 interacts with and regulates SARM1 during axonal injury. PNAS. 2022; 119(47):e2203824119. PMID: 36375051
Li Y, Lei Z, Ritzel RM, He J, Li H, Choi HMC, Lipinski MM# and Wu J#. Impairment of autophagy after spinal cord injury potentiates neuroinflammation and motor function deficit in mice. Theranostics. 2022; 12(12):5364-5388. PMID: 35910787
Hegdekar N, Lipinski MM# and Sarkar C#. N-acetyl-L-leucine treatment attenuates neuronal cell death and suppresses neuroinflammation after traumatic brain injury in mice. Sci Reports. 2021; 11(1):9249. PIMID: 33927281
Sarkar C, Jones JW, Hegdekar N, Thayer JA, Kumar A, Faden AI, Kane MA, Lipinski MM. PLA2G4A/cPLA2-mediated lysosomal membrane damage leads to inhibition of autophagy and neurodegeneration after brain trauma. Autophagy. 2020; 16(3):466-485. PMID: 31238788.
Thayer JA, Awad O, Hegdekar N, Sarkar C, Tesfay H, Burt C, Zeng X, Feldman RA, Lipinski MM. The PARK10 gene USP24 is a negative regulator of autophagy and ULK1 protein stability. Autophagy. 2020;16(1):140-152. PMID: 30957634
Li Y, Jones JW, M C Choi H, Sarkar C, Kane MA, Koh EY, Lipinski MM#, Wu J#. cPLA2 activation contributes to lysosomal defects leading to impairment of autophagy after spinal cord injury. Cell Death Dis. 2019; 10(7):531. PMID:31296844
Liu S, Li Y, Choi HMC, Sarkar C, Koh EY, Wu J# and Lipinski MM#. Lysosomal damage after spinal cord injury causes accumulation of RIPK1 and RIPK3 proteins and potentiation of necroptosis. Cell Death & Disease. 2018; 9:476. PMID: 29686269
Additional Publication Citations
Other Highlighted Publications
Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, Yuan J. A Genome-wide Analysis Reveals Mechanisms Modulating Autophagy in Normal Brain Aging and in Alzheimer's Disease. PNAS. 2010;107(32):14164-9. PMID: 20660724.
Lipinski MM, Hoffman G, Ng A, Zhou W, Py BF, Hsu E, Liu X, Eisenberg J, Liu J, Blenis J, Xavier RJ, A Genome-wide SiRNA Screen Reveals Multiple MTORC1 Independent Signaling Pathways Regulating Autophagy Under Normal Nutritional Conditions. Developmental Cell. 2010; 18(6):1041-52. PMID: 20627085.
Complete List of Published Work:
Research Interests
Function and Mechanisms of Autophagy in Neurotrauma & Neurodegeneration
Autophagy is a catabolic process mediating the turnover of bulk cytoplasmic constituents including organelles and protein aggregates in a lysosome-dependent manner. It is necessary for cellular homeostasis and protects organisms from a variety of diseases, including neurodegeneration and aging. Recent data also implicate autophagy in regulation of inflammation, with high levels of autopahgy generally associated with anti-inflammatory, and low leveld with pro-inflammatory polarization. Accumulation of autophagosomes has been observed following traumatic brain injury (TBI) and spinal cord injury (SCI), but its mechanisms and function in those contexts remain unknown. We use in vivo and in vitro models to examine the role of autophagy after TBI and SCI, and to delineate the molecular mechanisms involved.
Our data demonstrate that although autophagosomes accumulate in the brain and spinal cord after TBI and SCI, respectively, autophagic degradation cannot proceed to completion. The block of autophagy after CNS injury is apparent in several cell types, including neurons, microglia and monocytes infiltrating the brain and spinal cord after injury. We are using multiple approaches including cell-type specific reporter and gene knock out mice, imaging, transcriptomics, cell type and orgenalle-specific lipidomics/proteomics, behavioral assays and in vitro molecular approaches to invesitgate the mechanisms leading to inhibition of autopahgy and the effects of this inhibition in different cell types. We are also investigating the role of autopahgy in development of neurodegenerative diseases following exposure to TBI.
Our long-term goal is to define novel target molecules and pathways for safe and effective modulation of autophagy as a treatment against neurodegeneration induced by both acute (trauma) and chronic (neurodegenerative diseases) causes.
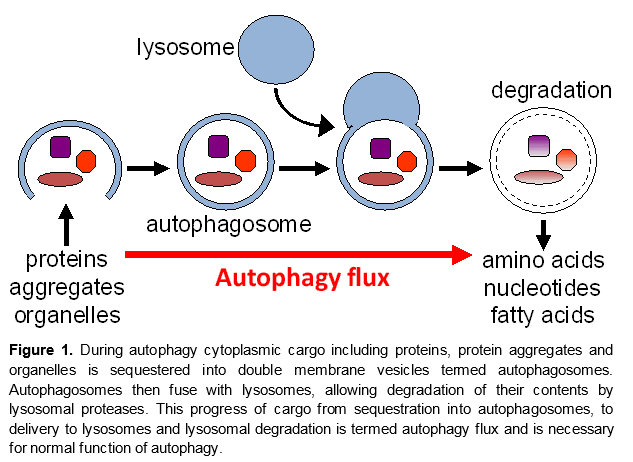
Current Projects:
Our current work focuses on the mechanisms and the role perturbation of autophagy plays in neurodegeneration and neuroinflammation after brain injury and during brain aging. We are also investigating whether inhibition of autophagy and lysosomal function contributes to increased prevalence of neurodegenerative diseases in patients with history of TBI. Finally, we are looking at the interaction between autophagy and lipid metabolism and their contribution to neuroinflammation after TBI and during brain aging.
Mechanisms of autophagy dysfunction after traumatic brain injury: We are using transgenic mice and in vitro models to determine the mechanisms leading to lysosomal damage and disruption of autophagy flux following traumatic brain injury (TBI). We are focusing on the effects of inhibition of autophagy on neuronal cell death and neuroinflammation after TBI as well as testing whether restoration of autophagy can be used as a treatment to improve functional outcomes after brain trauma.
Role of autophagy impairment in connecting history of TBI to development of neurodegenerative diseases: Inhibition of autophagy is known to contribute to development of many age-related neurodegenerative diseases. Our data indicate that autopahgy is also inhibited in neurons and microglia after TBI. We hypothesize that the TBI-induced inhibition of auophagy may contribute to increased predisposition to neurodegenrative disease in individuals with history of TBI. To test this hypothesis we are comparing brain aging trajectories in mice with wnd without exposure to TBI.
Effects of autophagy disruption on neuronal cell death and functional recovery after spinal cord injury: In collaboration with Dr. Junfang Wu (Anesthesiology) and Dr. Eugene Koh (Orthopaedics) we are using transgenic mouse mice and in vitro models to determine the influence of disruption of lysosomal function and autophagy flux on apoptotic and non-apoptotic neuronal cell death mechanisms following spinal cord injury (SCI). We are also investigating potential involvement of inhibition of autophagy in axonal damage observed after SCI and testing whether enhancing autophagy may have beneficial effects on functional outcomes after SCI.
Interaction between autophagy and lipid metabolism in neurotrauma and brain aging: Brain has higher lipid content than any other tissue except adipose and contains up to 25% of body cholesterol. Recent data indicate that lipid environment can affect cellular processes, including autophagy-lysosomal function. Coversely, autophagy is involved in cellular lipid metabolism, including lipid efflux. We are using transgenic mouse movels and in vitro studies to dissect interaction between lipid metabolism and autophagy after TBI and during brain aging.
Grants and Contracts
Active Grant Support:
- Cell-type and organelle-specific multi-omics platform for the study of brain aging
NIH R33 AG076858 (MPI: Marta Lipinski / Maureen Kane / Michael Cummings)
04/01/2022 – 03/31/2026
- The Function and Mechanisms of Autophagy in Spinal Cord Injury
NIH 2R01 NS094527 (MPI: Junfang Wu / Marta Lipinski)
04/01/2022 – 03/31/2027
- Dysregulation of autophagy-lysosomal function links TBI to late-onset neurodegeneration
NIH R01 NS091218 (PI: Marta Lipinski)
04/01/2020 - 03/31/2025
- Development of diagnostic biomarkers for determination of traumatic brain injury
FDA / CERSI 3U01FD005946 (MPI: Maureen Kane / Marta Lipinski / Jace Jones / Chinmoy Sarkar)
9/1/2019 - 8/31/2023
In the News
Professional Activity
Professional Societies
- Member, Society for Neuroscience
- Member, National Neurotrauma Society
- Member, American Society for Cell Biology
Editorial Service
- Associate Editor, Autophagy
Links of Interest
Lab website: https://www.lipinski-lab.org/
Research Gate: https://www.researchgate.net/profile/Marta-Lipinski
Anesthesiology research website: https://www.medschool.umaryland.edu/Anesthesiology/Research/
Lab Members
Current Lab Members
- Chinmoy Sarkar, PhD, Assistant Professor
- Amir Mehrabani, Graduate Student (PhD Program in Molecular Medicine)
- Sage Thapa, Graduate Student (PhD Program in Molecular Medicine)
- Liv Pettyjohn-Robin, Lab Assistant
From left: Shuo Liu, Niv Hegdekar, Marta Lipinski, Julia Thayer and Chinmoy Sarkar
Former Lab Members
- Nivedita Hegdekar, PhD, Graduate Student, Program in Biochemistry, 2017-2022; Postdoctoral Fellow, 2022-2023
- Current Position: Strategy Consultant, IQVIA, Boston, MA
- Harry Choi, PhD, Postdoctoral Fellow, 2017-2022
- Current Position: Postdoctoral fellow, Japan
- Sabrina Bustos, Lab technician, 2020-2022
- Current Position: Student, College of Osteopathic Medicine, Liberty University, Lynchburg, VA
- Denisha Odie, MS Student, Master of Health Sciences Program, 2021
- Current Position: Applying to Law School
- Julia Thayer, PhD, Graduate Student, Program in Toxicology, 2015-2019; Postdoctoral Fellow, 2019-2020
- Current Position: Postdoctoral Fellow, NIH, Bethesda, MD
- Josh Ostovitz, MS Student, Cellular & Molecular Biomedical Science Masters Program, 2017-2019
- Current position: Research Technician, Center for Translational Research In Imaging, University of Maryland School of Medicine, Baltimore, MD
- Shuo Liu, MD-PhD, Postdoctoral Fellow, 2012-2017
- Current Position: Resident in Pathology, University of South Alabama, Mobile, AL
- Deepika Philkana, MS Student, Cellular and Molecular Biomedical Science Masters Program, 2015-2016
- Current position: Research Associate-II, Sera Care Life Sciences, Gaithesburg, MD
- Prarthana Ravishankar, MS Student, Cellular and Molecular Biomedical Science Masters Program, 2014-2015
- Current position: Special Project Associate, Mayo Clinic, Jacksonville, FL
Undergraduate and High School Summer Interns
- 2023: Rachel Katz (UMCP), Sarah Griffith (UMCP)
- 2022: Brandon Kim (HS)
- 2021: Anna Choi (HS)
- 2019: Nicholas Leahy (UMCP)
- 2018: Delwin Suraj (UMCP), Tatiana Sabirzhanov (HS)
- 2017: Cameran Burt (UMCP), Martin Ejeh (UMCP), Serena Debesai (HS), Tatiana Sabirzhanov (HS)
- 2016: Henok Tesfay (UMCP), Cameran Burt (UMCP), Annika Schaefer (Vanderbilt U), Harshal Shah (HS)
- 2015: Malik Richberg (HS)
- 2014: Steven Kurapaty (UMCP), Julie Ethridge (UMCP)
- 2013: Julie Ethridge (UMCP)
Collaborators
Collaborators at UMB
- Junfang Wu, MD-PhD, Departament of Anesthesiology & Shock, Trauma and Anesthesiology Research (STAR) Center
- Eugene Koh, MD-PhD, Departmetn of Orthopaedics
- Ricardo Feldman, PhD, Department of Microbiology & Immunology
- Ola Awad, PhD, Department of Microbiology & Immunology
- Maureen A. Kane, PhD, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy
- Jace W. Jones, PhD, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy
Collaborators Outside UMB
- Michael Cummings, PhD, Center for Bioinformatics and Computational Biology, University of Maryland College Park, MD
- Kim Finley, PhD, San Diego State University, San Diego, CA
- Xianmin Zeng, PhD, Buck Institute for Research on Aging, Novato, CA
