Research
Lung Transplantation
Alex (Zhongcheng) Mei, MD, Mojtaba Taheri, MD, and Yizhan Guo, MD
Despite over 30 years of clinical experience with lung transplantation long-term survival lags behind other solid organs due to high rates of both acute and chronic rejection. Mechanistic studies using murine models have helped advance our understanding of lung allograft-specific immune responses and offer the potential for the development of novel methods of immunoregulation. Specifically, and unlike most other solid organs, the lung relies on pro-inflammatory feedback loops for both establishing and maintaining tolerance. We have previously described that CD8+ T cells, long considered deleterious to solid organ allograft survival, play a critical role in lung graft acceptance. We have similarly uncovered that lung-resident eosinophils, also previously considered to be a detrimental pro-rejection cell population, shape CD8+ T cell fate to induce tolerance. In fact, the eosinophil-CD8+ T cell feedback loop plays a crucial role in the maintenance of graft health. Further understanding of such previously unrecognized regulatory mechanisms offers the opportunity for the development of lung-allograft specific immunosuppression. Such work is now actively being pursued in our laboratory.
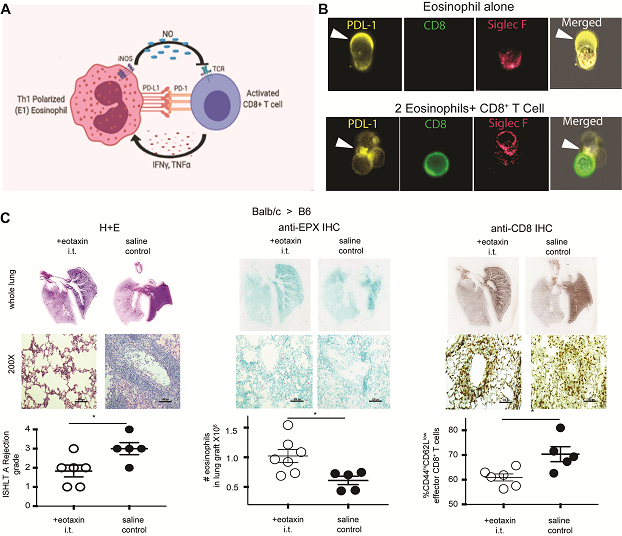
(A) Unlike most other organs, the lung establishes a unique regulatory feedback loop consisting of CD8+ T cell production of IFN-γ/TNF-α (B) PDL-1 mediated interactions play a key role in eosinophil:CD8+ T cell synapse formation. (C) Mobilization of eosinophils into the lung graft through intratracheal administration of eotaxin and IL-5 decreases rejection, and CD8+ T cell effector differentiation. Data reproduced from this paper.
Lung Cancer
Lung Cancer Immunotherapy Research
Anirban Bannerjee, PhD and Dongge Li, MD
While natural killer (NK) cells play an important role in controlling hematologic malignancies our laboratory has provided data that they also present a major immunologic barrier to the development of certain solid tumors, such as lung cancer (1). We and others have demonstrated that robust or weak NK function correlates with resistance or susceptibility to lung cancer, respectively. However, the reasons for such differences in NK function and cancer susceptibility have remained unknown. Recent work from our laboratory has demonstrated that the process of NK education or “licensing” during their development can facilitate the control of lung cancer in mice. Such data paves the way for novel therapies directed at “arming” the immune system to control lung cancer which are now being pursued in our laboratory.

Mice with licensed or educated NK cells control the growth of both translated (A) as well as carcinogen-induced lung cancer (B) and have better lytic ability for lung cancer cell lines (C). Modified from this paper.









---Edited-200x250.jpg)



