October 17, 2025 | Deborah Kotz
Study Led by UM School of Medicine Faculty at the Center for Vaccine Development and Global Health Met Safety Targets and Demonstrated Efficacy
 Malaria remains one of the leading causes of death among children in sub-Saharan Africa, claiming more than 600,000 lives each year worldwide with limited efficacy in currently available treatments and vaccines. Now a new early-stage clinical trial found that a novel monoclonal antibody demonstrated dose dependent efficacy against the malaria parasite with minimal side effects.
Malaria remains one of the leading causes of death among children in sub-Saharan Africa, claiming more than 600,000 lives each year worldwide with limited efficacy in currently available treatments and vaccines. Now a new early-stage clinical trial found that a novel monoclonal antibody demonstrated dose dependent efficacy against the malaria parasite with minimal side effects.
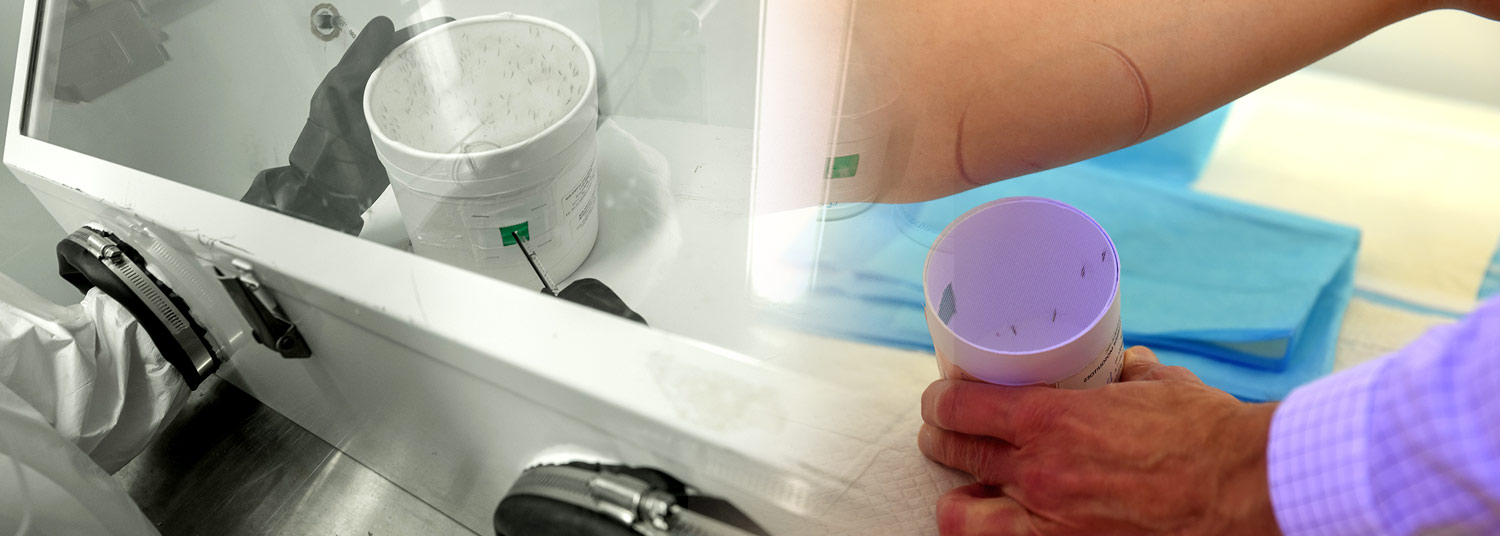
Researchers at the University of Maryland School of Medicine’s Center for Vaccine Development and Global Health (CVD) conducted the trial in healthy volunteers who were exposed, in a controlled manner, to bites, from mosquitos infected with the malaria parasite.
Results were recently published in The Lancet. The researchers conducted the trial, sponsored by the Gates Medical Research Institute, with funding from the Gates Foundation..
 “Despite major advances, malaria continues to devastate families and communities across Africa,” said study lead author Kirsten E. Lyke, MD, Professor of Medicine at UM School of Medicine and principal investigator at CVD. “This new monoclonal antibody could transform how we prevent malaria in young children and pregnant women. Unlike vaccines that may require multiple doses or boosters, a single injection of a long-acting antibody could provide immediate, months-long protection. It’s a fundamentally different way to stop infection before it starts.”
“Despite major advances, malaria continues to devastate families and communities across Africa,” said study lead author Kirsten E. Lyke, MD, Professor of Medicine at UM School of Medicine and principal investigator at CVD. “This new monoclonal antibody could transform how we prevent malaria in young children and pregnant women. Unlike vaccines that may require multiple doses or boosters, a single injection of a long-acting antibody could provide immediate, months-long protection. It’s a fundamentally different way to stop infection before it starts.”
Monoclonal antibodies (mAbs) are laboratory-made protein clones that mimic the body’s natural immune defenses. MAM01 targets a highly conserved region of the Plasmodium falciparum circumsporozoite protein — a protein on the parasite’s outer surface — to block infection before it reaches the bloodstream.
 The Phase 1, double-blind, placebo-controlled trial enrolled 38 healthy adults aged 18 to 50 with no prior malaria exposure. Participants received one dose of MAM01 or a placebo, and were then exposed to mosquitos carrying malaria, several months after dosing. This was done under carefully controlled conditions known as a challenge study. After the malaria challenge, none of the participants who received the highest dose of the monoclonal antibody developed infection, compared to all the participants in the placebo group.
The Phase 1, double-blind, placebo-controlled trial enrolled 38 healthy adults aged 18 to 50 with no prior malaria exposure. Participants received one dose of MAM01 or a placebo, and were then exposed to mosquitos carrying malaria, several months after dosing. This was done under carefully controlled conditions known as a challenge study. After the malaria challenge, none of the participants who received the highest dose of the monoclonal antibody developed infection, compared to all the participants in the placebo group.
“These early results suggest that this monoclonal antibody have the potential to provide reliable protection against malaria, which continues to disproportionately affect children who live in low and middle-income countries,” said study co-author Matthew B. Laurens, MD, MPH, Professor of Pediatrics and Director of the Malaria International Clinical Trials Unit at CVD. “This is an important proof-of-concept for the field and a step forward for health equity.”
 No treatment-related serious adverse events occurred.
No treatment-related serious adverse events occurred.
“Testing of this preventive treatment has already started in young children in Uganda, based on the promising results from the first trial conducted here,” said UM School of Medicine Dean Mark T. Gladwin, MD, who is also the Vice President for Medical Affairs at the University of Maryland, Baltimore (UMB), and the John Z. and Akiko K. Bowers Distinguished Professor.
Added James Campbell, MD, MS, Interim Director of the Center for Vaccine Development and Global Health: “This study represents real hope for millions of children at risk. CVD has been a global leader in malaria research for more than 50 years, and these findings advance our mission to eliminate this disease through innovative science.”
About the Center for Vaccine Development and Global Health
The University of Maryland’s Center for Vaccine Development and Global Health (CVD) is internationally recognized for its pioneering work on malaria, cholera, typhoid, and other infectious diseases. For more than five decades, CVD scientists have partnered with communities around the world to develop, test, and deliver life-saving vaccines that improve global health outcomes.
About the University of Maryland School of Medicine
The University of Maryland School of Medicine, established in 1807 as the first public medical school in the U.S., continues today as one of the fastest growing, top-tier biomedical research enterprises in the world. The School has nearly $500 million total research funding, 46 departments, centers, and institutes, more than 2,200 student trainees and over 3,000 faculty members, including notable members of the National Academy of Medicine. As the largest public medical school in the DC/MD/VA region, faculty-physicians are working to help patients manage chronic diseases like obesity, cancer, heart disease and addiction, while also working on cutting-edge research to address the most critical generational health challenges. In 2024, the School ranked #12 among public medical schools and #27 among all medical schools for R&D expenditures by the National Science Foundation. With a $1.3 billion total operating budget, the School partners with the University of Maryland Medical Center to serve nearly 2 million patients annually. The School's global reach extends around the world with research and treatment facilities in 33 countries. In Maryland, the School of Medicine is spearheading new initiatives in AI and health computing and partnering with the University of Maryland BioPark to develop new medical technologies and bioengineering ventures. For more information, visit medschool.umaryland.edu.
Contact
Deborah Kotz
dkotz@som.umaryland.edu