Academic Title:
Professor
Primary Appointment:
Medicine
Location:
UMMC N9E17
Phone (Primary):
410-706-2191
Fax:
410-706-3260
Education and Training
- 1984: Bachelor of Science (B.S.) Liceo Scientifico Statale "F. D’Assisi" Rome, Italy
- 1991: Medical Doctor (M.D.) degree University of Rome "La Sapienza" Italy. Graduated with 110/110 summa cum laude, on the basis of public defense of thesis entitled: "Metastatic phenotype: Growth factor dependence and Integrin expression"
- 1997: Ph.D. in Medical Biotechnology (Ph.D.). University of L'Aquila, Italy. Public defense of a dissertation entitled: "Transcriptional regulation of hematopoietic cell proliferation and differentiation".
Highlighted Publications
Perrotti D, Bonatti S, Trotta R, Martinez R, Skorski T, Salomoni P, Grassilli E, Lozzo RV, Cooper DR, Calabretta B. TLS/FUS, a pro-oncogene involved in multiple chromosomal translocations, is a novel regulator of BCR/ABL-mediated leukemogenesis. EMBO J. 1998 Aug 3;17(15):4442-55.
Perrotti D, Cesi V, Trotta R, Guerzoni C, Santilli G, Campbell K, Iervolino A, Condorelli F, Gambacorti-Passerini C, Caligiuri MA, Calabretta B. BCR-ABL suppresses C/EBPalpha expression through inhibitory action of hnRNP E2. Nat Genet. 2002 Jan;30(1):48-58.
Iervolino A, Santilli G, Trotta R, Guerzoni C, Cesi V, Bergamaschi A, Gambacorti-Passerini C, Calabretta B, Perrotti D. hnRNP A1 nucleocytoplasmic shuttling activity is required for normal myelopoiesis and BCR/ABL leukemogenesis. Mol Cell Biol. 2002 Apr;22(7):2255-66.
Trotta R, Vignudelli T, Candini O, Intine RV, Pecorari L, Guerzoni C, Santilli G, Byrom MW, Goldoni S, Ford LP, Caligiuri MA, Maraia RJ, Perrotti D, Calabretta B. BCR/ABL activates mdm2 mRNA translation via the La antigen. Cancer Cell. 2003 Feb;3(2):145-60. PMID: 12620409.
Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004 Jun 1;103(11):4010-22. Review.
Additional Publication Citations
Peer Reviewed Journal Articles (selected from 107)
Perrotti D, Bonatti S, Trotta R, Martinez R, Skorski T, Salomoni P, Grassilli E, Lozzo RV, Cooper DR, Calabretta B. TLS/FUS, a pro-oncogene involved in multiple chromosomal translocations, is a novel regulator of BCR/ABL-mediated leukemogenesis. EMBO J. 1998 Aug 3;17(15):4442-55.
Perrotti D, Cesi V, Trotta R, Guerzoni C, Santilli G, Campbell K, Iervolino A, Condorelli F, Gambacorti-Passerini C, Caligiuri MA, Calabretta B. BCR-ABL suppresses C/EBPalpha expression through inhibitory action of hnRNP E2. Nat Genet. 2002 Jan;30(1):48-58.
Iervolino A, Santilli G, Trotta R, Guerzoni C, Cesi V, Bergamaschi A, Gambacorti-Passerini C, Calabretta B, Perrotti D. hnRNP A1 nucleocytoplasmic shuttling activity is required for normal myelopoiesis and BCR/ABL leukemogenesis. Mol Cell Biol. 2002 Apr;22(7):2255-66.
Trotta R, Vignudelli T, Candini O, Intine RV, Pecorari L, Guerzoni C, Santilli G, Byrom MW, Goldoni S, Ford LP, Caligiuri MA, Maraia RJ, Perrotti D, Calabretta B. BCR/ABL activates mdm2 mRNA translation via the La antigen. Cancer Cell. 2003 Feb;3(2):145-60. PMID: 12620409.
Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004 Jun 1;103(11):4010-22. Review.
Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, Mao H, Chang JS, Galietta A, Uttam A, Roy DC, Valtieri M, Bruner-Klisovic R, Caligiuri MA, Bloomfield CD, Marcucci G, Perrotti D. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005 Nov;8(5):355-68.
Notari M, Neviani P, Santhanam R, Blaser BW, Chang JS, Galietta A, Willis AE, Roy DC, Caligiuri MA, Marcucci G, Perrotti D. A MAPK/HNRPK pathway controls BCR/ABL oncogenic potential by regulating MYC mRNA translation. Blood. 2006 Mar 15;107(6):2507-16.
Chang JS, Santhanam R, Trotta R, Neviani P, Eiring AM, Briercheck E, Ronchetti M, Roy DC, Calabretta B, Caligiuri MA, Perrotti D. High levels of the BCR/ABL oncoprotein are required for the MAPK-hnRNP-E2 dependent suppression of C/EBPalpha-driven myeloid differentiation. Blood. 2007 Aug 1;110(3):994-1003.
Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, Druker BJ, Cortes J, Marcucci G, Chen CS, Verrills NM, Roy DC, Caligiuri MA, Bloomfield CD, Byrd JC, Perrotti D. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007 Sep;117(9):2408-21.
Eiring AM, Neviani P, Santhanam R, Oaks JJ, Chang JS, Notari M, Willis W, Gambacorti-Passerini C, Volinia S, Marcucci G, Caligiuri MA, Leone GW, Perrotti D. Identification of novel posttranscriptional targets of the BCR/ABL oncoprotein by ribonomics: requirement of E2F3 for BCR/ABL leukemogenesis. Blood. 2008 Jan 15;111(2):816-28.;
Eiring AM, Harb JG, Neviani P, Garton C, Oaks JJ, Spizzo R, Liu S, Schwind S, Santhanam R, Hickey CJ, Becker H, Chandler JC, Andino R, Cortes J, Hokland P, Huettner CS, Bhatia R, Roy DC, Liebhaber SA, Caligiuri MA, Marcucci G, Garzon R, Croce CM, Calin GA, Perrotti D. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010 Mar 5;140(5):652-65.
Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010 Jul;120(7):2254-64. Review.
Perrotti D, Harb JG. BCR-ABL1 kinase-dependent alteration of mRNA metabolism: potential alternatives for therapeutic intervention. Leuk Lymphoma. 2011 Feb;52 Suppl 1:30-44. Review.
Trotta R, Ciarlariello D, Dal Col J, Mao H, Chen L, Briercheck E, Yu J, Zhang J, Perrotti D, Caligiuri MA. The PP2A inhibitor SET regulates granzyme B expression in human natural killer cells. Blood. 2011 Feb 24;117(8):2378-84.
Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 2013 May;14(6):e229-38. Review.
Oaks JJ, Santhanam R, Walker CJ, Roof S, Harb JG, Ferenchak G, Eisfeld AK, Van Brocklyn JR, Briesewitz R, Saddoughi SA, Nagata K, Bittman R, Caligiuri MA, Abdel-Wahab O, Levine R, Arlinghaus RB, Quintas-Cardama A, Goldman JM, Apperley J, Reid A, Milojkovic D, Ziolo MT, Marcucci G, Ogretmen B, Neviani P, Perrotti D*. Antagonistic activities of the immunomodulator and PP2A-activating drug FTY720 (Fingolimod, Gilenya) in Jak2-driven hematologic malignancies. Blood. 2013 Sep 12;122(11):1923-34.
Harb JG, Neviani P, Chyla BJ, Ellis JJ, Ferenchak GJ, Oaks JJ, Walker CJ, Hokland P, Roy DC, Caligiuri MA, Marcucci G, Huettner CS, Perrotti D. Bcl-xL anti-apoptotic network is dispensable for development and maintenance of CML but is required for disease progression where it represents a new therapeutic target. Leukemia. 2013 Oct;27(10):1996-2005.
Neviani P, Harb JG, Oaks JJ, Santhanam R, Walker CJ, Ellis JJ, Ferenchak G, Dorrance AM, Paisie CA, Eiring AM, Ma Y, Mao HC, Zhang B, Wunderlich M, May PC, Sun C, Saddoughi SA, Bielawski J, Blum W, Klisovic RB, Solt JA, Byrd JC, Volinia S, Cortes J, Huettner CS, Koschmieder S, Holyoake TL, Devine S, Caligiuri MA, Croce CM, Garzon R, Ogretmen B, Arlinghaus RB, Chen CS, Bittman R, Hokland P, Roy DC, Milojkovic D, Apperley J, Goldman JM, Reid A, Mulloy JC, Bhatia R, Marcucci G, Perrotti D. PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J Clin Invest. 2013 Oct 1;123(10):4144-57.
Walker CJ, Oaks JJ, Santhanam R, Neviani P, Harb JG, Ferenchak G, Ellis JJ, Landesman Y, Eisfeld AK, Gabrail NY, Smith CL, Caligiuri MA, Hokland P, Roy DC, Reid A, Milojkovic D, Goldman JM, Apperley J, Garzon R, Marcucci G, Shacham S, Kauffman MG, Perrotti D. Preclinical and clinical efficacy of XPO1/CRM1 inhibition by the karyopherin inhibitor KPT-330 in Ph+ leukemias. Blood. 2013 Oct 24;122(17):3034-44.
Neviani P, Perrotti D. SETting OP449 into the PP2A-Activating Drug Family. Clin Cancer Res. 2014 Apr 15;20(8):2026-8. PMID: 24634375.
Research Interests
Dr. Perrotti is a member of the Experimental Therapeutics Program within the University of Maryland Marlene and Stewart Greenebaum Cancer Center Program in Oncology. Dr. Perrotti's research centers on the identification of novel genes and proteins that are important regulators of hematopoietic stem can progenitor cell self-renewal and/or survivals and targets in cancer treatment.
Dr. Perrotti’s research has dealt with normal and malignant hematology since 1993. His interest has been focused for the past two decades on understanding the molecular mechanisms responsible for the emergence, maintenance and progression of leukemias with particular emphasis on Chronic Myelogenous Leukemia (CML). The ultimate goal of Dr. Perrotti's research is to find molecular targets useful for the development of new therapeutic drugs that will improve outcome of patients resistant to tyrosine kinase inhibitor-based therapies and eradicate the disease at stem cell level. Dr.
Perrotti's research (reviewed in the articles cited below) has generated paradigm shifts in the way scientists look at potential way to approach basic and translational science.
Dr. Perrotti's research results from successful collaborations with national and international clinical and basic science investigators, and has been supported by NCI and DOD grants since the time of Dr. Perrotti's first faculty academic appointment. Dr. Perrotti is also a former Scholar of the Leukemia and Lymphoma Society.
- Perrotti D, Neviani P. From mRNA metabolism to cancer therapy: chronic myelogenous leukemia shows the way. Clin Cancer Res. 2007 Mar 15;13(6):1638-42.
- Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120(7):2254-64
- Perrotti D, Harb JG. BCR-ABL1 kinase-dependent alteration of mRNA metabolism: potential alternatives for therapeutic intervention. Leuk Lymphoma. 2011 Feb; 52 Suppl 1:30-44.
- Perrotti D. and Neviani P. Protein phosphatase 2A: a target for anticancer therapy.. Lancet Oncol. 2013; 14(6):e229-38.
Professional Activity
- 1985-1990: Pre-graduation training. Molecular Oncogenesis Lab. - Regina Elena Cancer Inst.,Rome, Italy.
- 1990-1991: Research Assistant, Molecular Oncogenesis Lab., Regina Elena Cancer Institute of Rome, Italy
- 1991-1992: Visiting Scientist, International Institute of Genetics and Biophysics (IIGB-C.N.R.) Naples, Italy.
- 1993-1997: PhD student employed at Thomas Jefferson University, Philadelphia PA
- 1997-1999: Postdoctoral Researcher, Kimmel Cancer Inst., Thomas Jefferson University, Philadelphia, PA.
- 1999-2002: Research Instructor, Kimmel Cancer Institute, Thomas Jefferson University, Philadelphia, PA
- 2003-2008: Assistant Professor (Full-time; Tenure-track), MVIMG, OSUCCC, The Ohio State University
- 2008-2013: Associate Professor (Tenure), Dept. Mol. Virol., Immunol. and Medical Genetics, and Member of the Comprehensive Cancer Center, The Ohio State University, Columbus OH 43210.
- 2010-pres.: Honorary Senior Lecturer, The Imperial College of London, London UK.
- 2013-pres.: Professor (Tenure), Dept. of Medicine and Dept. of Biochemistry and Molecular Biology; Director Basic Hematologic Research and Member of the Experimental Therapeutic Program, Greenebaum Cancer Center, The University of Maryland School of Medicine, Baltimore MD.
Lab Techniques and Equipment
National and International Collaborators and Lab Members:

Current Perrotti's Lab Members:
- Rossana Trotta, Ph.D (Assistant Professor)
- Giovannino Silvestri, Ph.D (Postdoctoral Fellow)
- Lorenzo Stramucci, Ph.D. (Postdoctoral fellow)
- Justine YU, B.S. (Graduate Student, Molecular Medicine Program)
Key Research Achievements
1. Altered mRNA metabolism in cancer
Dr. Perrotti and co-workers were the first to report that oncogene-dependent alterations of RNA metabolism significantly contribute to leukemia emergence, maintenance and progression.
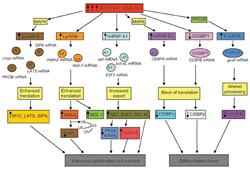
They discovered that the BCR-ABL1 oncoprotein, product of the t(9;22) translocation, exerts its leukemogenic potential and promotes CML disease progression in part by altering expression and function of specific RNA binding proteins (Eiring AM et al., Blood 2008) and microRNAs (Eiring et al., Cell 2010) essential for the correct proliferation, survival and differentiation of myeloid progenitors. Specifically, they reported that increased levels of BCR-ABL1 kinase activity, as those found in blast crisis CML, leads to differentiation arrest through hnRNP E2-dependent translational inhibition of C/EBPa expression (Perrotti D. et al., Nature Genet. 2002; Chang JS. et al Blood 2007); functional inactivation of p53 tumor suppressor activity through La-dependent translational enhancement of MDM2 expression (Trotta R. et al., Cancer Cell 2003); aberrant activation of mitogenic and survival signals via hnRNP K-dependent translational stimulation of Myc expression (Notari M. et al., Blood 2006); and to hnRNP A1/SET-dependent inhibition of PP2A tumor suppressor activity, a necessary step for full activation of BCR/ABL oncogenic signalosome and for positive autoregulation of wild type and tyrosine kinase inhibitor-resistant oncogene expression and activity in both leukemic stem/progenitor cells (Iervolino et al., Mol. Cell Biol. 2002; Neviani P. et al., Cancer Cell 2005; Harb J.G. et al. Leukemia 2013). Furthermore, they reported that such aberrant pathways could be therapeutically targeted by XPO1 inhibitors (e.g. KPT-330) in patients with advanced Ph+ leukemias (Walker C. et al., Blood 2013).
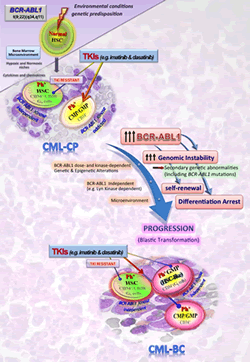
2. Block of myeloid maturation in blast crisis CML
Dr. Perrotti and co-workers dissected the molecular mechanism responsible for differentiation arrest in myeloid CML blast crisis (CML-BC).
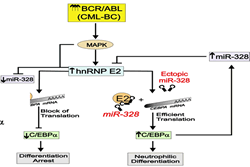
Specifically they show that, in most of clinical cases, block of differentiation in CML-BC is a BCR-ABL1 kinase-dependent effect that depends on increased BCR-ABL1 expression/activity that, upon constitutively activating the MEK1/ERK pathway, leads to increased expression of hnRNP E2 which, in turn, inhibits translation of C/EBPa, the major regulator of granulocytic differentiation, upon binding a C-rich element within the CEBPA mRNA 5’UTR (Perrotti et al., Nat. Genet. 2002; Chang et al., Blood 2007). Moreover, they showed that suppression of granulocytic differentiation in myeloid CML-BC requires downregulation of miR-328, a microRNA positively regulated by C/EBPa and capable of directly restraining the translation inhibitory effect of hnRNP E2 and restoring differentiation of primary myeloid CML-BC progenitors (Eiring AM. et. al., Cell 2010).
3. MicroRNA decoy activity.
Dr. Perrotti and co-workers were the first to describe the microRNA decoy activity.
The showed that microRNAs not only can suppress gene expression upon binding the mRNA 3’UTR but they also can simultaneously interact with RNA binding proteins (decoy activity) in a seed-sequence independent manner and interfere with their ability to regulate mRNA processing, stability and translation (Eiring AM et al., Cell 2010; Balkhi MY. et al., Sci. Signal 2013). This article received printed and/or audio/visual press coverage from international and national websites/journals, including Science (AAAS), Nature Medicine, Nature Reviews of Cancer (Nature Press), Leukemia and Lymphoma Society and a Cell Previews “MicroRNAs: From Decay to DecoybyMichaela Beitzinger, Gunter Meister. Cell 140(5):612, 2010”. It was also highlighted as Cell Video Abstract http://www.youtube.com/watch?v=mZ-iUoNB6LQ
4. Reactivating the tumor suppressor PP2A with PADs.
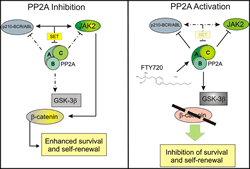
Dr. Perrotti and co-workers were the first to show that inactivation of the tumor suppressor protein phosphate 2A (PP2A) is a common and therapeutically druggable event during leukemogenesis.
Specifically, they reported that PP2A is inactivated in chronic and blastic phase CML and other oncogenic kinase-driven hematopoietic disorders (e.g. Ph-negative MPNs, Ph+ ALL, and AML) through the activity of its endogenous inhibitors (e.g., SET), which are aberrantly regulated by oncogenic tyrosine (e.g. BCR-ABL1, Jak2, KIT, FLT3-ITD) kinases (Neviani P. et al., Cancer Cell 2005; Neviani et. al J. Clin. Invest. 2007; Samantha et al., Oncogene 2009; Roberts KG., et al. Cancer Res. 2010; Oaks, JJ. et al., Blood 2013). Interestingly, SET-dependent inhibition of PP2A activity is a BCR-ABL1 kinase-dependent and –independent effect in leukemic progenitor and stem cells, respectively; however, in both cell population it requires JAK2 activity. Moreover, we were the first to show that genetic (SET downregulation or PP2Ac overexpression) or pharmacologic restoration of PP2A enzymatic activity by PP2A-activating drugs (PADs; e.g. forskolin, FTY720 and their derivatives) can impair leukemic stem and progenitor cell survival both in vitro and in animal models without harming normal hematopoiesis or inducing adverse/toxic effects. In collaboration with other investigators, we also showed that true PADs reactivate PP2A upon interaction and sequestration of the physiological PP2A inhibitors (e.g. SET). Mechanistically, we reported that reactivation of PP2A simultaneously leads to inactivation/degradation of the oncogenic kinases (e.g. BCR-ABL1) and direct inactivation of BCR-ABL1 kinase-dependent and –independent mitogenic, survival and self-renewal signals in both stem and progenitor leukemic cells (Neviani et al., J. Clin. Invest 2007; Neviani et al., J. Clin. Invest 2013).
This work led to a) the discover by other groups that PP2A is inactivated in almost all hematologic malignancies and in several solid cancers where this tumor suppressors is not genetically altered, and b) to the generation of other PADs with clear anti-cancer activity and very low toxicity profile (reviewed in Perrotti and Neviani, Cancer. Metastasis Rev. 2008; Perrotti and Neviani, Lancet Oncol. 2013; Neviani and Perrotti, Clin. Cancer Res. 2014). This work resulted in outstanding publications, which also received strong press coverage (over 100 national/international websites and journals), public media attention in over 40 US states, several scientific commentaries/articles (e.g. including Nature Reviews of Cancer, AAAS Science updates, Eurekalert, Reuters Health, Medpage Today, Medscape-WebMD, Federal Drug Administration news, Oncology Today, Oncolink, AACI and American Society of Clinical Oncology), and three national and/or international patents (US patent 8,633,161 - 2014; PCT/US2013/043521; and PCT/US2007/00046049). This work was also highlighted in the Annual Report of the Dept. of the Army-CDMRP-CML to the US Congress.
5. Oncogene-addiction vs. oncogene-requirement.
Dr. Perrotti and co-workers were the first to determine that an oncogenic tyrosine kinase (e.g. BCR-ABL1) can exert its oncogenic potential regardless of its kinase activity, and that this might be cell context-dependent.
They reported that persistence of leukemic HSCs in BM requires inhibition of the tumor suppressor PP2A and expression — but not activity — of the BCR-ABL1 oncogene. Specifically, they showed that BCR-ABL1 is acting as scaffold protein to recruit and maintain an active oncogenic signalosome in stem cells while CML progenitors mostly relies on, and become addicted to BCR-ABL1 kinase activity. In fact, TKI-resistant CML quiescent HSCs showed increased levels of BCR-ABL1, but very low kinase activity. BCR-ABL1 expression, but not kinase function, was required for recruitment of JAK2, activation of a JAK2/ß-catenin survival/self-renewal pathway, and inhibition of PP2A (Neviani et al., J. Clin. Invest 2013).