Division Chief
Daniel G. Maluf, MD
With the ever-growing need for organ, eye, tissue, and other transplants, research and innovation in transplantation continue to be a key factor to healing and saving more lives. Our Division research and clinical teams are poised to be leaders in the transplant field and to achieve success through innovative approaches at each area of the transplant environment.
The Division of Transplantation team provides patient access to quality of care and innovative approaches to patients all our partners in the field of transplantation. The broad areas of basic research are cellular and molecular immunology of migration and lymph node structure and bioengineering for the manipulation of immunity. The clinical and translation research focuses on clinical and translational studies in renal and liver transplantation.
Areas of expertise include biomarker discovery and mechanistic studies, including single RNAseq studies in liver and kidney transplantation and gene editing in areas of pump perfusion, renal chronic allograft dysfunction and ischemia reperfusion injury.
Jonathan Bromberg, MD, PhD
Jonathan Bromberg, MD, PhD
Dr. Bromberg has been involved continuously in basic cellular and molecular transplant immunology for more than 25 years and has been continuously funded for the entire time. His basic research has always focused on T cell immunobiology, and for more than 15 years has also focused on issues of migration, trafficking, secondary lymphoid organ structure and function, and lymphatic structure and function, as well as how these processes and structures influence T cell immunity and T cell tolerance in models of cardiac transplantation and pancreatic islet transplantation.
Dr. Bromberg has also maintained an active clinical practice in solid organ transplantation and is thus constantly exposed to the problems of patients and their immune systems, including cellular and humoral rejection, opportunistic infections, chronic viral disease, autoimmune organ failure, and immunosuppression medication side effects.
His basic research and clinical interests are especially well suited to complement and inform each other, and to keep each aspect of his professional life current and relevant.
Stephen Gray, MD, MPH
Stephen Gray, MD, MPH
Diagnostic and Therapeutic Applications of Microarrays in Liver Transplantation, a Multicenter Study (INTERLIVER)
Given the limitations of the conventional opinion-based histology and laboratory-developed tests such as DSA and C4d staining, future biopsy assessment will require centralized molecular tests (the Molecular Microscope®), with evidence-based interpretation. The system will incorporate strengths of each platform. This will be achieved by calculating the pre-biopsy probabilities and by comparison of the unknown biopsy with its “nearest neighbors” in the growing Reference Set. There will be three assessments: Conventional alone (local standard of care); Molecular Microscope® (MMDx™ alone); Integrated MMDx™ and conventional assessments.This will be accomplished by the INTERLIVER study, which will incorporate our experiences from the INTERCOMEX study of kidney transplant biopsies (clinicaltrials.gov NCT01299168) and INTERHEART study of heart transplant biopsies (clinicaltrials.gov NCT02670408). We will demonstrate a real-time value of MMDx™ reports, study the effect of therapy on clinical and biopsy parameters, time course of disease states, and propose a new integrated classification of disease states. Our ultimate goal is a diagnostic system that is:Probabilistic; Updatable with new data; Consensus-seeking; Acknowledges prior probabilities
- Sponsor: Alberta Transplant Applied Genomics Centre
Raphael Meier, MD, PhD
Raphael Meier, MD, PhD
Genetic determinants of glucose homeostasis in pancreas donors for islet transplantation for type 1 diabetes
The aim of this project is to identify genetic variants associated with glucose metabolism using a genome-wide association study in donors whose pancreases were used for islet transplantation for type 1 diabetic patients. We will use the Cardio-MetaboChip which assesses 196,725 SNPs related to metabolic syndrome components. In preliminary experiments, we studied donor serum levels of fibroblast growth factor 21 (FGF-21) to test our genotype dataset. FGF-21 is produced by the liver and promotes insulin synthesis by β-cells. This allowed us to identify a number of loci associated with donor FGF-21 levels. Given these promising preliminary results, we plan to organize our project as follows: Part I: identification of the genetic variants associated with direct in vitro (static glucosestimulated insulin secretion) and in vivo β-cell function (graft survival). Part II: proceed to an in vitro assessment of candidate genes using β-cells, myocytes, and hepatocytes to test the functional influence of the genetic variants identified in Part I. Part III: test in vivo the functional influence of the genetic variants identified as functionally relevant in vitro in Part II. We will use conditional knockout mice fed with high fat/high sucrose diet and control mice to study the effect on glucose homeostasis.
- Sponsor: Diabetes Research Connection
Genetically-engineered mesenchymal stem cells (MSCs) to enhance anti-inflammatory cytokine secretion to treat alcoholic/nonalcoholic fatty liver disease.
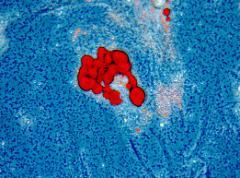
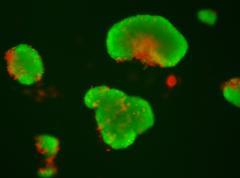
This project investigates MSC transplantation as a treatment for chronic liver diseases. We were able to demonstrate that encapsulated MSCs can produce anti-inflammatory IL-1Ra and reduce liver fibrosis in two different mice models. These studies allowed to set up efficiently encapsulation models using different type of cells. We are currently working on developing bone marrow- and adipose-derived MSC transplantation as anti-inflammatory vectors in a preclinical model of steatohepatitis induced by high fat / high sucrose diet. Our current project includes three parts: Part I: To engineer human bone marrow-derived MSCs to selectively enhance their production of selected cytokines previously identified to inhibit inflammation and liver fibrosis. Part II: To study the potential protective effects of transplanted engineered and encapsulated human bone marrow-derived MSCs in vitro and in vivo. Part II: To initiate a safety and efficacy phase-I/II clinical trial to treat patients with nonalcoholic steatohepatitis who have no other therapeutic options.
- Sponsor: University of Maryland, Baltimore
Josue Alvarez-Casas, MD
Josue Alvarez-Casas, MD
may have a clinical trial with OrganOX
Daniel G. Maluf, MD
Daniel G. Maluf, MD
Just received a corporate research agreement
Joseph Scalea, MD
Joseph Scalea, MD
Does research
Georgios Vrakas, MD
Georgios Vrakas, MD
No funding, does research



