May 21, 2020 | Deborah Kotz

Study Designed for Hospitalized Patients on Ventilators
Researchers at the University of Maryland School of Medicine (UMSOM) have begun testing an experimental stem cell therapy developed by Mesoblast Limited to treat hospitalized COVID-19 patients with moderate to severe acute respiratory distress syndrome (ARDS) who are on ventilators to help them breathe. The trial, which is being conducted at the University of Maryland Medical Center (UMMC) and additional sites across the U.S, will involve a total of 300 patients randomized to receive either the drug remestemcel-L or a placebo in addition to the recommended standard of care to manage severe COVID-19 infections. The first patient in this national trial was treated at UMMC.
The research, funded by Mesoblast, an Australian regenerative medicine company, is designed to determine whether the drug reduces the risk of death within 30 days after the onset of treatment and whether it reduces the number of days from a ventilator to recovery.
“This stem cell therapy is a potential new therapy in our treatment arsenal to battle COVID-19,” said Sunjay Kaushal, MD, PhD, Professor of Surgery at the University of Maryland School of Medicine and Chief of the University of Maryland Congenital Heart Disease Outreach Program. “There is an urgent need to find new life-saving therapies for our sickest COVID-19 patients who are suffering from ARDS and require ventilators. We are eager to see whether remestemcel-L can reduce mortality in these patients.”
Remestemcel-L is an experimental stem cell therapy developed for various inflammatory conditions, and it is believed to reduce inflammation by reducing the production of pro-inflammatory chemicals called cytokines. COVID-19 patients often become very ill from an escalated immune response, referred to as a cytokine storm, creating high levels of inflammation that can be fatal.
The trial will include other hospitals in the University of Maryland Medical System that have COVID-19 patients who meet the criteria. "As we extend the reach of this study to other UMMS hospitals, we affirm our commitment as an academic health system to explore all avenues to improve the health of the people we serve," said Mohan Suntha, MD, MBA, President and Chief Executive Officer of the University of Maryland Medical System. "Our hope is that research efforts like this and others we are pursuing in the midst of a novel coronavirus pandemic will help save lives."
Enrollment is expected to complete within three to four months, with interim analyses planned which could result in stopping the trial early if the drug appears to be working well or not working at all. Potential adverse effects of the treatment include rejection of the cells by the body and other immune system reactions.
“The University of Maryland School of Medicine continues to take the lead in researching innovative new therapies including stem cell therapies, and we are part of an urgent national mission to provide potentially lifesaving options to patients,” said Dean E. Albert Reece, MD, PhD, MBA, who is also Executive Vice President for Medical Affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor, University of Maryland School of Medicine. “Our faculty are engaged in a myriad of research efforts to identify and test innovative techniques to help in the fight against this horrible pandemic.”
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 45 academic departments, centers, institutes, and programs; and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.2 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically based care for nearly 2 million patients each year. The School of Medicine has more than $540 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 student trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of nearly $6 billion and an economic impact more than $15 billion on the state and local community. The School of Medicine faculty, which ranks as the 8th highest among public medical schools in research productivity, is an innovator in translational medicine, with 600 active patents and 24 start-up companies. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
About the University of Maryland Medical Center
The University Of Maryland Medical Center (UMMC) is comprised of two hospital campuses in Baltimore: the 800-bed flagship institution of the 14-hospital University of Maryland Medical System (UMMS) — and the 200-bed UMMC Midtown Campus, both academic medical centers training physicians and health professionals and pursuing research and innovation to improve health. UMMC’s downtown campus is a national and regional referral center for trauma, cancer care, neurosciences, advanced cardiovascular care, women's and children's health, and has one of the largest solid organ transplant programs in the country. All physicians on staff at the downtown campus are clinical faculty physicians of the University of Maryland School of Medicine. The UMMC Midtown Campus medical staff is predominately faculty physicians specializing in diabetes, chronic diseases, behavioral health, long term acute care and an array of outpatient primary care and specialty services. UMMC Midtown has been a teaching hospital for 140 years and is located one mile away from the downtown campus. For more information, visit www.umm.edu.
Contact
Deborah Kotz
Director of Media Relations
Office of Public Affairs & Communications
University of Maryland School of Medicine
o: 410-706-4255
c: 410-804-0054
t: @debkotz2
Related stories

Tuesday, December 20, 2022
COVID Vaccines Prevented 3 Million Deaths in the U.S., New Analysis Finds
In the two years since the first COVID-19 vaccines were given to patients in the U.S., the vaccines had the cumulative effect of preventing 18 million hospitalizations and 3 million deaths. That is based on a new modeling analysis conducted by a researcher at the University of Maryland School of Medicine (UMSOM) and her colleagues. Results of the analysis were published by the Commonwealth Fund.

Wednesday, February 16, 2022
Study Shows New Drug Combination More Effective Against SARS-CoV-2
Researchers at the University of Maryland School of Medicine (UMSOM) and University of Pennsylvania Perelman School of Medicine have identified a powerful combination of antivirals to treat COVID-19. The researchers showed that combining the experimental drug brequinar with either of the two drugs already approved by the U.S. Food and Drug Administration for emergency use, remdesivir or molnupiravir, inhibited growth of the SARS-CoV-2 virus in human lung cells and in mice. Their findings suggest that these drugs are more potent when used in combination than individually.

Wednesday, January 26, 2022
Trial Co-led by University of Maryland School of Medicine Scientist Confirms Safety of “Mix-and-Match” COVID-19 Vaccine Booster Dosing
A University of Maryland School of Medicine (UMSOM), Center for Vaccine Development and Global Health (CVD), expert is co-leading an ongoing study that was pivotal in recommending adults and teens receive booster COVID-19 shots of their choosing starting in fall 2021. The preliminary clinical trial results, reported today in The New England Journal of Medicine, found that is safe and effective to receive boosters that are the same or a different one from the person’s primary vaccine(s).

Thursday, December 16, 2021
Novavax COVID-19 Vaccine Found to be Safe and Effective in Phase 3 Trial Conducted by UM School of Medicine Researchers
An investigational COVID-19 vaccine made by Novavax was found to be 90 percent effective at preventing COVID-19 illness, according to results from a Phase 3 clinical trial published today in the New England Journal of Medicine. The University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health served as one of the trial sites, and Karen Kotloff, MD, Professor of Pediatrics at UMSOM, served as Co-Chair for the trial protocol.

Tuesday, December 14, 2021
UM School of Medicine’s Office of Public Affairs and Communications Honored with 2021 PRSA ‘Best In Maryland’ Award
The University of Maryland School of Medicine (UMSOM)'s Office of Public Affairs & Communications received the Public Relations Society of America (PRSA) 2021 “Best in Maryland Award,” the PRSA’s highest honor, for its COVID-19 Communications program. The award was given at the PRSA Maryland Gala, held on December 9, 2021.

Wednesday, September 08, 2021
UM School of Medicine Reaching Underserved Communities Through Grass Roots Efforts to Increase COVID-19 Vaccination Rates
In an effort to increase COVID-19 vaccination rates among children and families, and ultimately help bring the pandemic under control, the Department of Family & Community Medicine (DFCM) and the Department of Psychiatry at the University of Maryland School of Medicine (UMSOM) are partnering with key community and faith-based groups in Baltimore city to reach the most vulnerable and underserved communities. This partnership will also extend across Maryland, Delaware, Virginia, and West Virginia.

Wednesday, July 07, 2021
UM School of Medicine Researchers Develop Two Rapid Tests for COVID-19 Using Innovative Techniques
Researchers at the University of Maryland School of Medicine (UMSOM) have developed two rapid diagnostic tests for COVID-19 that are nearly as accurate as the gold-standard test currently used in laboratories. Unlike the gold-standard test, which extracts RNA and uses it to amplify the DNA of the virus, these new tests can detect the presence of the virus in as little as five minutes using different methods.

Wednesday, June 09, 2021
Global Study of Microbes in 60 Cities Finds Each Has Unique Fingerprint of Viruses and Bacteria
Each city has its own unique microbiome, a “fingerprint” of viruses and bacteria that serves as type of city profile, according to a new study from an international consortium of researchers that included a team from the University of Maryland School of Medicine (UMSOM). The international project, which sequenced and analyzed samples collected from public transit systems and hospitals in 60 cities around the world, was published today in the journal Cell.

Tuesday, March 16, 2021
New Study Finds Healthcare Settings Do Not Pose Added Risk Factor for Covid-19 Infection Spread Among U.S. Healthcare Personnel
Healthcare personnel who were infected with COVID-19 faced stronger risk factors outside of the workplace than in their hospital or healthcare settings. That is the finding of a new study published today in the Journal of the American Medical association's JAMA Network Open conducted by University of Maryland School of Medicine (UMSOM) researchers and colleagues at the Centers for Disease Control and Prevention (CDC) and three other universities.

Thursday, January 21, 2021
UM School of Medicine Hosted Media Availability for Ensuring Trust in COVID-19 Vaccine Event
On January 22, 2021 at 2 p.m., the University of Maryland School of Medicine (UMSOM) hosted Black faith-based leaders, COVID-19 research volunteers, and “America’s Doctor,” Anthony Fauci, MD. The event provided straight talk about fears, trust issues, and why we need our Black and Brown community to be a part of COVID-19 vaccine research.
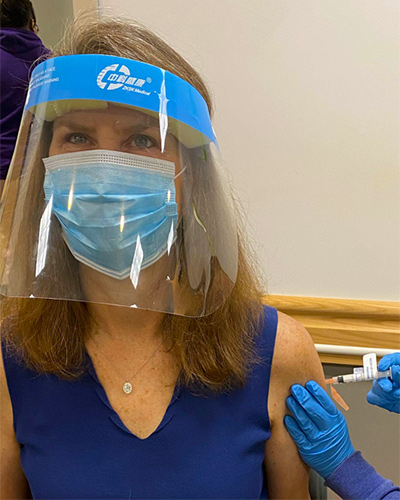
Tuesday, January 05, 2021
Dr. Kathleen Neuzil, World-renowned Leader in Vaccine Research, Receives Moderna Vaccine
Kathleen Neuzil, MD, MPH, FIDSA, the Myron M. Levine, MD, DTPH, Professor of Vaccinology and Director of the University of Maryland School of Medicine’s (UMSOM)’s Center for Vaccine Development and Global Health (CVD), received her first injection of the Moderna vaccine for COVID-19 on December 31. Researchers at the University of Maryland School of Medicine (UMSOM) played an integral part in the dedicated work that led to the U.S. Food and Drug Administration issuing an Emergency Use Authorization for the Moderna vaccine in December.

Tuesday, January 05, 2021
University of Maryland School of Medicine Begins Phase 3 Trial of Novavax COVID-19 Vaccine Candidate
Researchers at the University of Maryland School of Medicine (UMSOM) will participate in a Phase 3 clinical trial of an investigational COVID-19 vaccine to protect against SARS-CoV-2, the coronavirus causing COVID-19 that continues to impact millions of people around the world. The clinical trial will test the safety and effectiveness of NVX-CoV2373, being developed by U.S. biotechnology company, Novavax, Inc., based in Gaithersburg, MD.

Thursday, November 19, 2020
Promising Results Seen in Pfizer/BioNTech COVID-19 Vaccine After Phase 1 Trial by University of Maryland School of Medicine
Just six months after beginning a clinical development program that first enrolled here at the University of Maryland School of Medicine (UMSOM), Pfizer and BioNTech report interim results showing an mRNA COVID-19 vaccine had no serious safety concerns and has been found to be 95 percent effective in protecting individuals from COVID-19.

Friday, November 13, 2020
UM School of Medicine and School of Pharmacy Researchers Identify Promising New Compounds to Potentially Treat Novel Coronaviruses
Researchers at the University of Maryland School of Medicine (UMSOM) and School of Pharmacy (UMSOP) have discovered new drug compounds to potentially treat the novel coronavirus that causes COVID-19. The compounds disrupt the functioning of a protein complex inside human cells that the researchers discovered is critical for the replication and survival of coronaviruses. This finding could lead to the development of new broad-spectrum antiviral drugs that target viruses such as influenza, Ebola and coronaviruses, according to a new study published today in the Proceedings of the National Academy of Sciences (PNAS) journal.
Tuesday, June 16, 2020
UM School of Medicine Researchers Receive Federal Funding to Rapidly Test New Treatments for COVID-19
Researchers at the University of Maryland School of Medicine (UMSOM) will be partnering on an agreement funded by the federal government’s Defense Advanced Research Projects Agency (DARPA) to rapidly test hundreds of drugs, approved and marketed for other conditions, to see whether any can be repurposed to prevent or treat COVID-19. The compounds will be tested in studies using state-of-the-art technologies in the laboratory of coronavirus researcher Matthew Frieman, PhD., Associate Professor of Microbiology and Immunology at the University of Maryland School of Medicine. UMSOM will receive up to $3.6 million over the next year to fund this effort.

Tuesday, June 02, 2020
UM School of Medicine’s Institute of Human Virology Awarded Grants to Strengthen COVID-19 Response in Sub-Saharan Africa
The Center for International Health, Education and Biosecurity (Ciheb) at the University of Maryland School of Medicine’s Institute of Human Virology was awarded $4 million from the U.S. Centers for Disease Control and Prevention (CDC) to support coronavirus disease 2019 (COVID-19) response activities in Botswana, Nigeria, Malawi, and Mozambique.
Monday, June 01, 2020
In A COVID-19 World, Another Threat to the Health of Our Children
In the U.S., our children rarely fall ill to grave infections because they are protected by vaccines. Serious illnesses like measles, mumps, congenital rubella syndrome, chickenpox, diphtheria, tetanus, whooping cough, rotavirus diarrhea, hepatitis (A and B), polio and bacterial meningitis are all preventable through routine childhood vaccinations.

Thursday, May 28, 2020
UM School of Medicine Researchers Develop Experimental Rapid COVID-19 Test Using Innovative Nanoparticle Technique
Scientists from the University of Maryland School of Medicine (UMSOM) developed an experimental diagnostic test for COVID-19 that can visually detect the presence of the virus in 10 minutes. It uses a simple assay containing plasmonic gold nanoparticles to detect a color change when the virus is present. The test does not require the use of any advanced laboratory techniques, such as those commonly used to amplify DNA, for analysis. The authors published their work last week in the American Chemical Society’s nanotechnology journal ACS Nano.
Tuesday, May 05, 2020
UM School of Medicine is First in U.S. to Test Unique RNA Vaccine Candidate for COVID-19
In a significant development in the global effort to discover a safe and effective vaccine for COVID-19, researchers at the University of Maryland School of Medicine (UMSOM) became the first in the U.S. to begin testing experimental COVID-19 vaccine candidates developed by Pfizer and BioNTech. The research, funded by Pfizer Inc., will study the safety, efficacy, and dosing of an experimental mRNA -based vaccine.

Thursday, April 23, 2020
UM School of Medicine Researchers Test Remdesivir as Potential Therapy for COVID-19 Patients
Researchers at the University of Maryland School of Medicine (UMSOM) are testing the effectiveness of the investigational antiviral drug remdesivir in hospitalized adult patients with SARS-CoV-2 (COVID-19). The randomized controlled clinical trial is evaluating the safety and effectiveness of the drug, and it is part of a national study funded by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH).

Friday, April 10, 2020
University of Maryland School of Medicine Launches New Large Scale COVID-19 Testing Initiative
University of Maryland School of Medicine (UMSOM) Dean E. Albert Reece, MD, PhD, MBA, announced today the launch of a large-scale COVID-19 Testing Initiative that will significantly expand testing capability over the coming weeks, enabled by new funding of $2.5 million from the State of Maryland.

Tuesday, December 20, 2016
First U.S. Babies Treated in Unique Study of Adult Stem Cell Therapy for Congenital Heart Disease
In a first-in-children randomized clinical study, medical researchers at the University of Maryland School of Medicine (UM SOM) and the Interdisciplinary Stem Cell Institute (ISCI) at the University of Miami Miller School of Medicine have begun testing to see whether adult stem cells derived from bone marrow benefit children with the congenital heart defect hypoplastic left heart syndrome (HLHS).
