February 02, 2026 | Devin Fisher
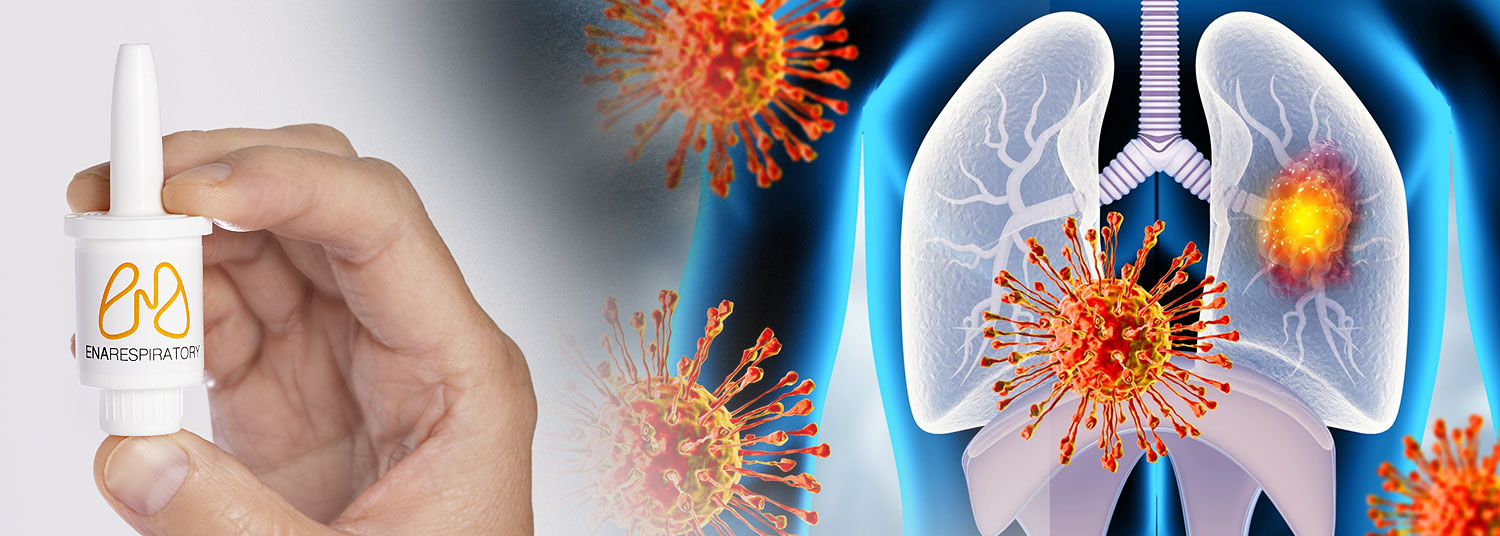
 Last year, at least one million people in the U.S. were hospitalized for respiratory virus illnesses like the flu or COVID-19, according to the Centers for Disease Control and Prevention. Many of these individuals were at higher risk of getting infections due to living or working around young children who contract more respiratory infections. A new clinical trial led by researchers at the University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health (CVD) will test a new experimental intranasal spray designed to boost immune defenses and reduce illness from respiratory viruses.
Last year, at least one million people in the U.S. were hospitalized for respiratory virus illnesses like the flu or COVID-19, according to the Centers for Disease Control and Prevention. Many of these individuals were at higher risk of getting infections due to living or working around young children who contract more respiratory infections. A new clinical trial led by researchers at the University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health (CVD) will test a new experimental intranasal spray designed to boost immune defenses and reduce illness from respiratory viruses.
The study is being conducted in collaboration with ENA Respiratory, the manufacturer of the novel therapy. The randomized double-blind Phase 2 trial aims to enroll 1,100 healthy adults ages 18 to 45 years who are at increased risk of upper respiratory virus infections due to exposure to young children or frequent close contact with others. Participants will be randomly assigned to receive the intranasal spray, called INNA-051, or a placebo spray to determine if INNA-051 is safe and works better than the placebo at boosting the immune response and preventing illness.
“This study represents a new approach to reducing illness from respiratory infections,” said Justin Ortiz, MD, Professor of Medicine and a respiratory illness specialist who is the UMSOM Principal Investigator for the ongoing trial. “Instead of targeting a single virus, INNA-051 strengthens the body’s early immune defenses, which may help mitigate disease caused by multiple respiratory pathogens.”
Resources for the Media:
Clinical trial footage, study investigator and study participant interviews.
 A non-vaccine, intranasal spray, INNA-051 works as a prophylactic drug designed to be taken weekly during cold and flu season. It is a TLR2/6 agonist, which works by priming the immune system’s first line of defense in an effort to accelerate the clearance of harmful germs from nasal passages before viruses can get a foothold in the body and cause an infection. INNA-051 is virus-agnostic, meaning that it can potentially help protect against a wide array of viruses, and acts in the nasal passages, the site of initial replication of common respiratory viruses, such as the flu, colds, and COVID-19. The study will investigate whether INNA-051 can reduce the severity or likelihood of illness during respiratory virus season.
A non-vaccine, intranasal spray, INNA-051 works as a prophylactic drug designed to be taken weekly during cold and flu season. It is a TLR2/6 agonist, which works by priming the immune system’s first line of defense in an effort to accelerate the clearance of harmful germs from nasal passages before viruses can get a foothold in the body and cause an infection. INNA-051 is virus-agnostic, meaning that it can potentially help protect against a wide array of viruses, and acts in the nasal passages, the site of initial replication of common respiratory viruses, such as the flu, colds, and COVID-19. The study will investigate whether INNA-051 can reduce the severity or likelihood of illness during respiratory virus season.
“This trial exemplifies CVD’s commitment to conducting innovative clinical research aimed at reducing the burden of infectious disease. INNA-051 has the potential to protect those most vulnerable to respiratory viral complications, including persons with chronic lung disease, heart disease, and diabetes,” said James Campbell, MD, MS, Professor of Pediatrics at UMSOM and Interim Director of the Center for Vaccine Development and Global Health.
 UMSOM Dean Mark T. Gladwin, MD, added: “Our researchers are aiming to demonstrate the exciting potential of TLR2/6 agonists to become the first prophylactic therapy against respiratory viral illness. Respiratory viruses continue to challenge our healthcare systems because they evade our body’s most fundamental immune barrier—the mucosal surfaces of our airways. A preventive approach that strengthens this frontline of defense has the potential to transform how we reduce viral transmission in the community and could help build resilience against future respiratory threats.”
UMSOM Dean Mark T. Gladwin, MD, added: “Our researchers are aiming to demonstrate the exciting potential of TLR2/6 agonists to become the first prophylactic therapy against respiratory viral illness. Respiratory viruses continue to challenge our healthcare systems because they evade our body’s most fundamental immune barrier—the mucosal surfaces of our airways. A preventive approach that strengthens this frontline of defense has the potential to transform how we reduce viral transmission in the community and could help build resilience against future respiratory threats.”
To learn more about this and other CVD clinical trials, visit CVDTrials.org.
About the Center for Vaccine Development and Global Health
Founded in 1974, the University of Maryland School of Medicine’s Center for Vaccine Development and Global Health is internationally recognized for its research in vaccine development, infectious disease, and clinical trials that have paved the way for lifesaving interventions around the world. The CVD’s mission is to reduce the global burden of infectious diseases through research, training, and public health impact.
About ENA Respiratory
ENA Respiratory is a clinical-stage pharmaceutical company tackling respiratory viral infections through the development of host defence enhancers which locally prime and boost the body’s natural first line of defence against invading pathogens. Being virus-agnostic, ENA’s approach offers a solution to protect against common and emerging respiratory viruses for which vaccines or direct-acting antivirals have limitations or do not exist.
The company’s lead product, INNA-051, is being developed as a convenient, once-a-week nasal dry powder product to reduce the impact of viral respiratory infections and prevent severe complications in at-risk populations, including the elderly, those with an underlying medical condition (including chronic lung conditions, diabetes, kidney disease, and cardiovascular disease) and individuals with occupational risk (e.g. first responders, military or essential services personnel). Headquartered in Melbourne, Australia, with operations in the USA, ENA’s Investors include Brandon Capital, Flu Lab, the Gates Foundation, the Minderoo Foundation, Stoic Venture Capital and Uniseed. The Company is partnered with the US COPD Foundation to support patient-focused clinical development of INNA-051 in COPD and has been awarded contracts from the U.S. Government. It is an alumni member of BLUE KNIGHT™, a joint initiative between Johnson & Johnson Innovation and BARDA designed to accelerate novel potential solutions for future pandemics. For more information, please visit https://enarespiratory.com
About the University of Maryland School of Medicine
The University of Maryland School of Medicine, established in 1807 as the first public medical school in the U.S., continues today as one of the fastest growing, top-tier biomedical research enterprises in the world. The School has nearly $500 million total research funding, 46 departments, centers, and institutes, more than 2,200 student trainees and over 3,000 faculty members, including notable members of the National Academy of Medicine. As the largest public medical school in the DC/MD/VA region, faculty-physicians are working to help patients manage chronic diseases like obesity, cancer, heart disease and addiction, while also working on cutting-edge research to address the most critical generational health challenges. In 2024, the School ranked #12 among public medical schools and #27 among all medical schools for R&D expenditures by the National Science Foundation. With a $1.3 billion total operating budget, the School partners with the University of Maryland Medical Center to serve nearly 2 million patients annually. The School's global reach extends around the world with research and treatment facilities in 33 countries. In Maryland, the School of Medicine is spearheading new initiatives in AI and health computing and partnering with the University of Maryland BioPark to develop new medical technologies and bioengineering ventures. For more information, visit medschool.umaryland.edu.
Contact
Devin Fisher
Devin.Fisher@som.umaryland.edu
Related stories

Wednesday, March 20, 2024
New Study Reveals Insights into Lack of Durability in COVID Antibody Response to Infections & Vaccines
Researchers at the Institute of Human Virology (IHV) at the University of Maryland School of Medicine published a new study in the Journal of Infectious Diseases investigating the short-lived antibody response following SARS-CoV-2, the virus that causes COVID.

Monday, March 29, 2021
Cancer Drug Lessens the Toxicity of a Protein from the Virus that Causes Covid-19, UM School of Medicine Study Finds
University of Maryland School of Medicine (UMSOM) researchers have identified the most toxic proteins made by SARS-COV-2—the virus that causes COVID-19 – and then used an FDA-approved cancer drug to blunt the viral protein’s detrimental effects. In their experiments in fruit flies and human cell lines, the team discovered the cell process that the virus hijacks, illuminating new potential candidate drugs that could be tested for treating severe COVID-19 disease patients. Their findings were published in two studies simultaneously on March 25 in Cell & Bioscience, a Springer Nature journal.

Thursday, January 21, 2021
UM School of Medicine Hosted Media Availability for Ensuring Trust in COVID-19 Vaccine Event
On January 22, 2021 at 2 p.m., the University of Maryland School of Medicine (UMSOM) hosted Black faith-based leaders, COVID-19 research volunteers, and “America’s Doctor,” Anthony Fauci, MD. The event provided straight talk about fears, trust issues, and why we need our Black and Brown community to be a part of COVID-19 vaccine research.

Tuesday, December 22, 2020
Largest Study of Its Kind Identifies Which COVID-19 Patients Face the Greatest Risk of Mortality During Hospitalization
Hospitalized COVID-19 patients have a greater risk of dying if they are men or if they are obese or have complications from diabetes or hypertension, according to a new study conducted by University of Maryland School of Medicine (UMSOM) researchers. In a study published in the journal Clinical Infectious Diseases, the researchers evaluated nearly 67,000 hospitalized COVID-19 patients in 613 hospitals across the country to determine the link between certain common patient characteristics and the risk of dying from COVID-19. Their analysis found that men had a 30 percent higher risk of dying compared to women of the same age and health status. Hospitalized patients who were obese, had hypertension or poorly managed diabetes had a higher risk of dying compared to those who did not have these conditions. Those aged 20 to 39 with these conditions had the biggest difference in their risk of dying compared to their healthier peers.

Thursday, October 22, 2020
New Landmark Study at UM School of Medicine Finds Aspirin Use Reduces Risk of Death in Hospitalized COVID-19 Patients
Hospitalized COVID-19 patients who were taking a daily low-dose aspirin to protect against cardiovascular disease had a significantly lower risk of complications and death compared to those who were not taking aspirin, according to a new study led by researchers at the University of Maryland School of Medicine (UMSOM). Aspirin takers were less likely to be placed in the intensive care unit (ICU) or hooked up to a mechanical ventilator, and they were more likely to survive the infection compared to hospitalized patients who were not taking aspirin, The study, published today in the journal Anesthesia and Analgesia, provides “cautious optimism,” the researchers say, for an inexpensive, accessible medication with a well-known safety profile that could help prevent severe complications.
Tuesday, June 16, 2020
UM School of Medicine Researchers Receive Federal Funding to Rapidly Test New Treatments for COVID-19
Researchers at the University of Maryland School of Medicine (UMSOM) will be partnering on an agreement funded by the federal government’s Defense Advanced Research Projects Agency (DARPA) to rapidly test hundreds of drugs, approved and marketed for other conditions, to see whether any can be repurposed to prevent or treat COVID-19. The compounds will be tested in studies using state-of-the-art technologies in the laboratory of coronavirus researcher Matthew Frieman, PhD., Associate Professor of Microbiology and Immunology at the University of Maryland School of Medicine. UMSOM will receive up to $3.6 million over the next year to fund this effort.

Monday, June 15, 2020
UM School of Medicine Researchers Help Identify Potent Antibody Cocktail with Potential to Treat COVID-19
Researchers at the University of Maryland School of Medicine (UMSOM) evaluated several human antibodies to determine the most potent combination to be mixed in a cocktail and used as a promising anti-viral therapy against the virus that causes COVID-19. Their research, conducted in collaboration with scientists at Regeneron Pharmaceuticals, was published today in the journal Science. The study demonstrates the rapid process of isolating, testing and mass-producing antibody therapies against any infectious disease by using both genetically engineered mice and plasma from recovered COVID-19 patients.

Tuesday, June 02, 2020
UM School of Medicine’s Institute of Human Virology Awarded Grants to Strengthen COVID-19 Response in Sub-Saharan Africa
The Center for International Health, Education and Biosecurity (Ciheb) at the University of Maryland School of Medicine’s Institute of Human Virology was awarded $4 million from the U.S. Centers for Disease Control and Prevention (CDC) to support coronavirus disease 2019 (COVID-19) response activities in Botswana, Nigeria, Malawi, and Mozambique.

Wednesday, September 12, 2018
UMSOM Influenza Expert Talks about Risk of Pandemics in Globally Connected World
Wilbur Chen, MD, Associate Professor of Medicine and a vaccine development specialist at the University of Maryland School of Medicine, discussed on WYPR’s “On the Record” how influenza and other diseases can now more easily spread around the world. In an interview with Radio Show Host Sheila Kast, Dr. Chen discussed how the Spanish influenza pandemic in 1918, which led to deaths between 50 and 100 million, actually spread at a slower pace than diseases today.

Thursday, October 05, 2017
UM SOM Researchers Identify Protein That Could Reduce Death and Improve Symptoms in Influenza and Other Infectious Diseases
A new study by researchers at the University of Maryland School of Medicine has identified an innovative strategy for treating influenza, and perhaps other infectious diseases as well. Scientists showed that a small protein called retrocyclin-101 (RC-101) could potentially improve the symptoms and mortality associated with the flu and possibly other types of infectious illness as well.
