Viral Vector Core
MISSION:
UMB Viral Vector Core (VVC) offers Adeno Associated Virus (AAV) and lentivirus-based gene delivery systems to be used in gene therapy for targeting a gene of interest (GOI) in in-intro and in-vivo studies. The VVC offers services for custom gene cloning and packaging in Adeno Associated Virus or lentivirus systems. The mission of this Core is to provide high quality viral vectors, reducing the time and cost for the investigators utilizing modern tools and technology. The Core offers free consultancy for gene construct designing strategy, discussing the research project, implementation, and troubleshooting.
The Center also supports collaborative efforts with researchers around the world on various topics.
SERVICES:
Viral Vector Core uses cutting-edge technologies to create and produce a variety of high-quality viral particles.
- Custom Gene cloning includes CRISPR elements for targeting gene activation, repression, or knockdown
- Conditional gene expression using tetracycline, inducible Systems, Cre-loxP, or using multiple combination approaches.
- Transfection grade DNA plasmid preparation
- AAV virus production
- Lentivirus production
- Pre-made ‘Stock virus’ with various range of serotypes
- Core also offers services to validate the virus using target qPCR and immunostaining, or western blot against your target gene or protein.
- Consultation for selection of gene and vector designing and implementation in your research.
- Feel free to contact us for a letter of support for your grant application.
ADENO-ASSOCIATED VIRUS (AAV) SERVICES:
- We provide research-grade and ultrapure, high quality AAV vectors with titers from 1x10E12 to 1x10E13 GC/ml, suitable for in-vivo transduction.
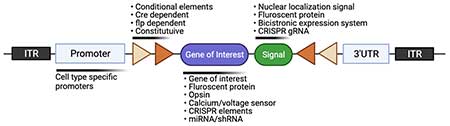
- We provide the service for custom gene cloning for AAV and lentivirus vector systems. We have the inventory of various promoter and fusion genes to design the custom constructs (Fig.1).
- We provide the service for highly purified and endotoxin-free DNA production used for virus production and in vitro transfection.
- We provide the pre-made ‘Stock virus’ with various AAV serotypes and lentivirus which is ready to use for your experiements. LENTIVIRUS SERVICES We offer the service for second and third-generation lentivirus particle production in a highly controlled and sterile environment following BSL2 guidelines. We determine the titers using qPCR using primers specific to the LTRs.The virus titer is guaranteed to be between 1x10E8 to 1x10E9 GC/ml.
LENTIVIRUS SERVICES
We offer the service for second and third-generation lentivirus particle production in a highly controlled and sterile environment following BSL2 guidelines. We determine the titers using qPCR using primers specific to the LTRs.The virus titer is guaranteed to be between 1x10E8 to 1x10E9 GC/ml.
AAV PRODUCTION AND QUALITY CONTROL
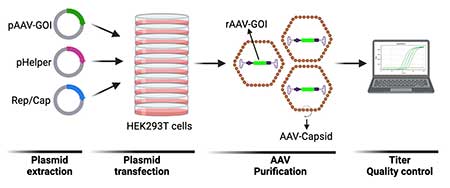
- We prepare the AAV virus using triple plasmid transfection in HEK-293 cells (Fig. 2). Before the transfection, custom DNA, serotype-specific plasmids expressing viral Rep/Cap proteins, and pHelper plasmid is produced by endotoxinfree Qiagen kit.
- rAAV particles are purified and concentrated by using Iodixanol (IDX) Gradient Ultracentrifugation.
- Purified virus preparations are subjected to quality control measures, including titer assays using qPCR (primers target the ITRs) and in vitro-transduction in HEK293T cells.
Diagrams
Click the graphics below to enlarge: