September 14, 2020 | Joanne Morrison
Researchers Are Vaccinating Participants to Test the COVID-19 mRNA Vaccine Developed by Moderna
 Researchers at the University of Maryland School of Medicine (UMSOM) have begun participation in the Phase 3 clinical trial of an investigational COVID-19 vaccine co-developed by scientists at Moderna Inc. and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). This clinical trial is a key step toward final approval of a vaccine to protect against the SARS-CoV-2 coronavirus that has impacted millions of people around the world.
Researchers at the University of Maryland School of Medicine (UMSOM) have begun participation in the Phase 3 clinical trial of an investigational COVID-19 vaccine co-developed by scientists at Moderna Inc. and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). This clinical trial is a key step toward final approval of a vaccine to protect against the SARS-CoV-2 coronavirus that has impacted millions of people around the world.
The vaccine trial is being conducted by researchers in UMSOM’s Center for Vaccine Development and Global Health (CVD). This is the first to be implemented under Operation Warp Speed, a multi-agency collaboration led by the U.S. Department of Health and Human Services (HHS), which aims to accelerate the development, manufacturing, and distribution of medical countermeasures for COVID-19.
Principal investigators for the clinical trial are Karen Kotloff, MD, Professor of Pediatrics, and Matthew Laurens, MD, MPH, Associate Professor of Pediatrics. The UMSOM site, expected to recruit several hundred participants, is part of the nationwide trial being conducted at 89 research sites that is expected to enroll 30,000 adults.
 “We urgently need safe and effective COVID-19 vaccines to control this pandemic. Vaccines can be rapidly given to protect whole populations, while treatments must be given to one person at a time after illness or exposure occurs," said Dr. Kotloff. "The Moderna mRNA-1273 vaccine has already shown promise in early testing in adults of all ages. This next trial will tell us whether it prevents COVID-19. It is critical that we include a diverse group of people in the trial, particularly those communities most impacted by this terrible virus, so the results will apply broadly to the population.”
“We urgently need safe and effective COVID-19 vaccines to control this pandemic. Vaccines can be rapidly given to protect whole populations, while treatments must be given to one person at a time after illness or exposure occurs," said Dr. Kotloff. "The Moderna mRNA-1273 vaccine has already shown promise in early testing in adults of all ages. This next trial will tell us whether it prevents COVID-19. It is critical that we include a diverse group of people in the trial, particularly those communities most impacted by this terrible virus, so the results will apply broadly to the population.”
This virus disproportionately affects older adults, people with unstable medical conditions, and racial and ethnic minorities, making it critical that the vaccine works well in those who need it most. UMSOM researchers are targeting people who have increased risk of exposure because of location or circumstance, such as occupation, to see whether the vaccine protects them. As part of that plan, the UMSOM researchers will enroll and vaccinate participants in Maryland’s hardest hit communities, including Langley Park and Baltimore. Researchers have partnered with CASA in Hyattsville, MD, the largest member-based Hispanic and immigrant organization in the mid-Atlantic region. Vaccinations will take place on campus in Baltimore and in Hyattsville/Langley Park.
“It is critical that we reach all communities that have been impacted by COVID-19,” said Dr. Laurens, highlighting the importance of UMSOM’s collaboration with CASA.
Moderna is leading the trial as the regulatory sponsor, and it is providing the investigational vaccine for the trial. The Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response and NIAID are providing funding support for the trial.
UMSOM is part of the NIAID-supported COVID-19 Prevention Network (CoVPN), which aims to enroll thousands of volunteers in large-scale clinical trials, testing a variety of investigational vaccines and monoclonal antibodies intended to protect people from COVID-19.
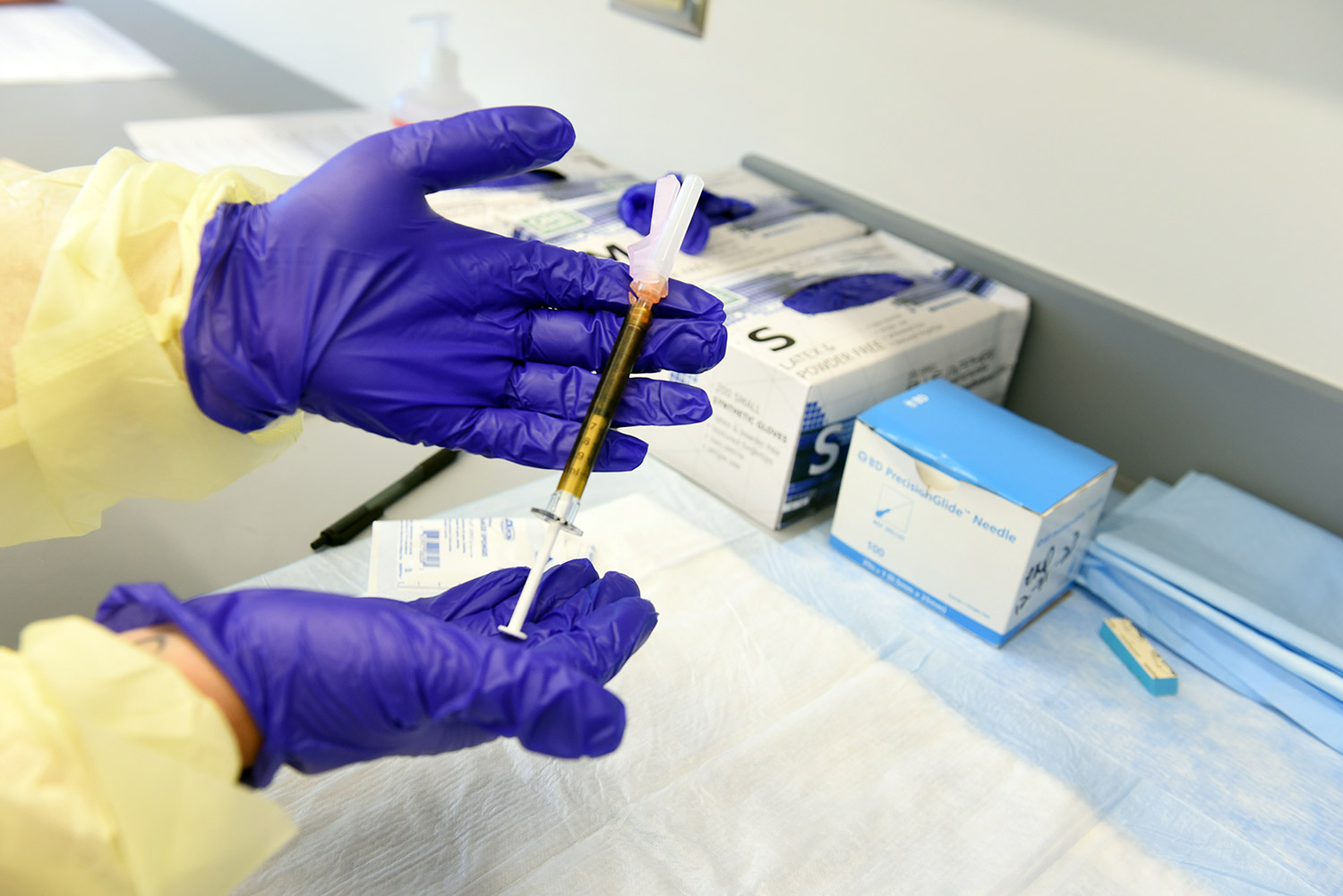
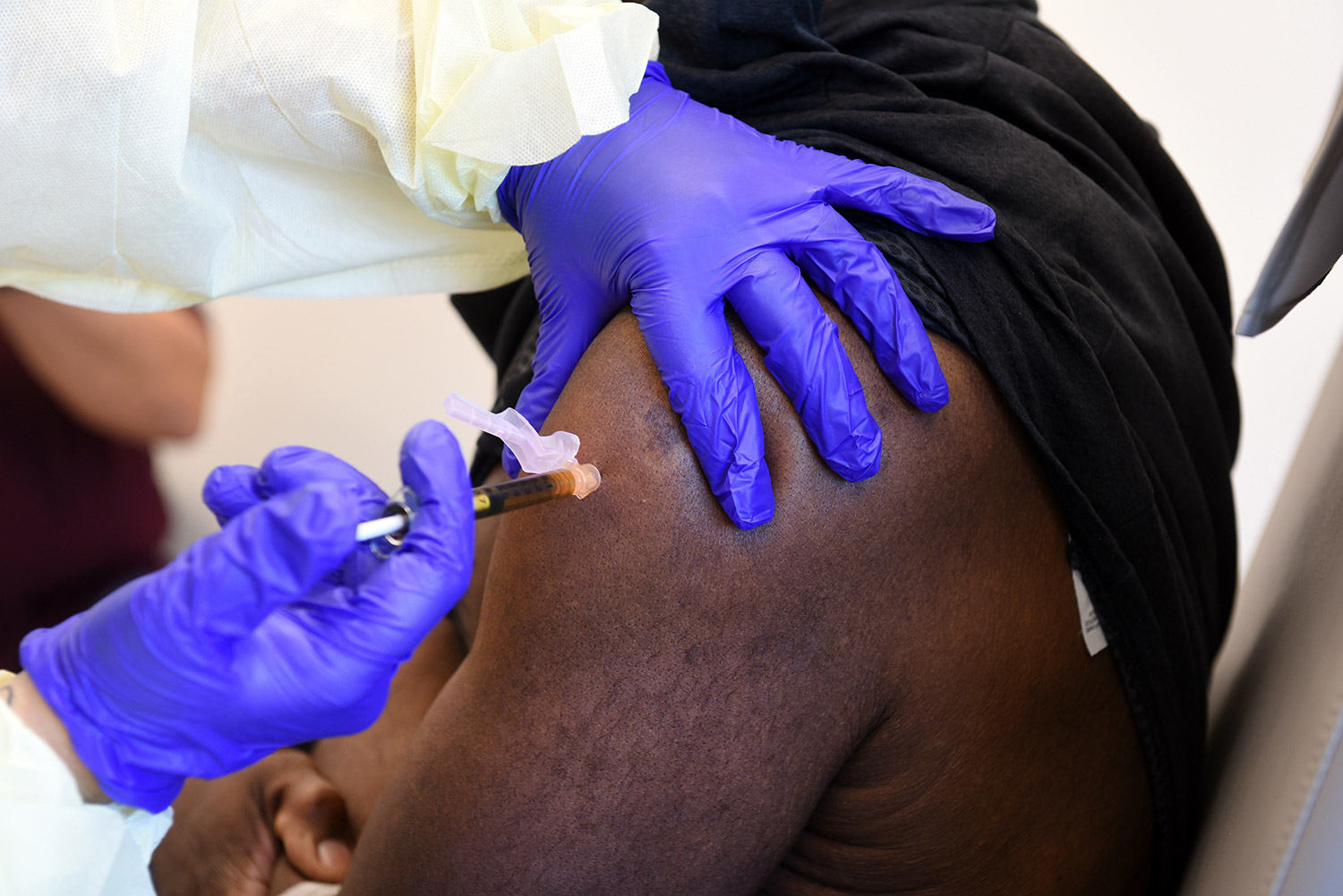
Trial volunteers will receive two intramuscular injections approximately 28 days apart. Participants will be randomly assigned to receive two injections of either the mRNA-1273 vaccine or salt water placebo. The trial is blinded, so the investigators and the participants will not know who is assigned to which group until after the study is over.
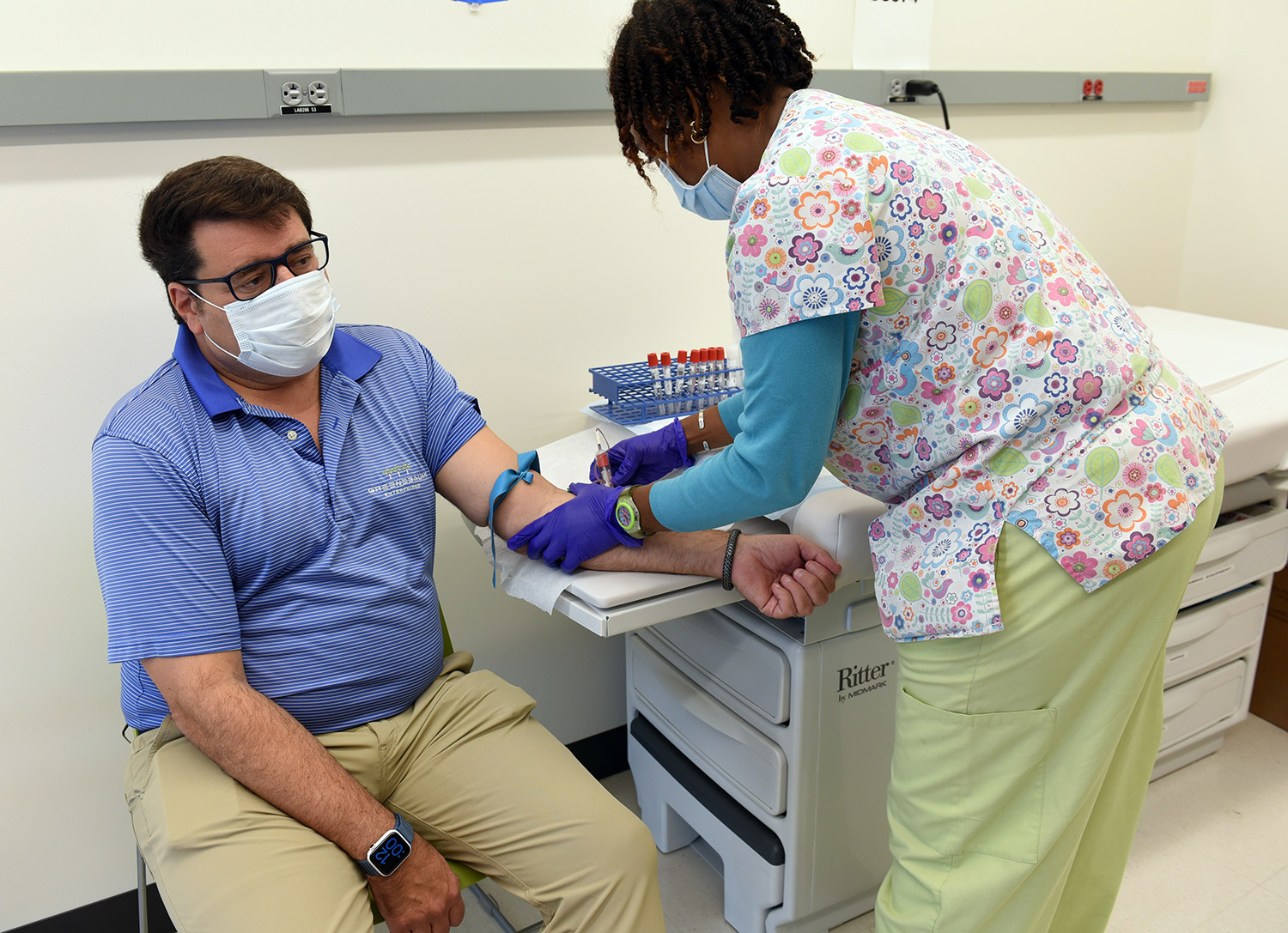
Participants will be asked to provide a nasopharyngeal swab and a blood sample at an initial screening visit, as well as at several time points after each vaccination and over the next two years after the second vaccination. Scientists will examine blood samples in the laboratory to better understand the immune responses to SARS-CoV-2 that provide protection.
 “Our team is contributing to the advancement of several promising COVID vaccine candidates. We are working diligently to ensure that the safety and performance of these vaccines is meticulously assessed,” said Kathleen Neuzil, MD, MPH, FIDSA, the Myron M. Levine, MD, DTPH Professor in Vaccinology and Director, Center for Vaccine Development and Global Health. Dr. Neuzil is a co-director of the CoVPN, which brings together experts from existing NIAID-supported clinical research networks to fight COVID-19.
“Our team is contributing to the advancement of several promising COVID vaccine candidates. We are working diligently to ensure that the safety and performance of these vaccines is meticulously assessed,” said Kathleen Neuzil, MD, MPH, FIDSA, the Myron M. Levine, MD, DTPH Professor in Vaccinology and Director, Center for Vaccine Development and Global Health. Dr. Neuzil is a co-director of the CoVPN, which brings together experts from existing NIAID-supported clinical research networks to fight COVID-19.
 The Moderna Phase 3 trial at CVD adds to COVID-19 research already underway at UMSOM. In May, CVD researchers Kathleen Neuzil, MD and Kirsten Lyke, MD, Professor of Medicine, led Phase 1 trials of mRNA vaccine constructs developed by Pfizer and BioNTech. Early results published this month in Nature showed that these Pfizer/BioNTech vaccines are well tolerated and have produced a robust immune response in healthy adult volunteers. Research on these Pfizer/BioNTech vaccines continues, as UMSOM researchers have now vaccinated a group of adults aged 65 and older as part of this study and are analyzing the results from that cohort.
The Moderna Phase 3 trial at CVD adds to COVID-19 research already underway at UMSOM. In May, CVD researchers Kathleen Neuzil, MD and Kirsten Lyke, MD, Professor of Medicine, led Phase 1 trials of mRNA vaccine constructs developed by Pfizer and BioNTech. Early results published this month in Nature showed that these Pfizer/BioNTech vaccines are well tolerated and have produced a robust immune response in healthy adult volunteers. Research on these Pfizer/BioNTech vaccines continues, as UMSOM researchers have now vaccinated a group of adults aged 65 and older as part of this study and are analyzing the results from that cohort.
About mRNA Vaccines
Both Moderna and Pfizer have chosen to develop mRNA vaccines – or messenger RNA vaccines – which direct the body’s cells to make the spike protein that projects from the surface of the virus. The vaccine is delivered inside of a lipid (fatty) envelope to strengthen the immune responses. This type of vaccine does not contain any part of the virus that could cause COVID-19. An advantage of an mRNA vaccine over a vaccine that directly injects the spike protein is the ability to rapidly produce large quantities of vaccine doses against a new pathogen.
At present, there is no Food and Drug Administration (FDA)-licensed mRNA vaccine. If continued research shows efficacy of this vaccine, it could serve as a key platform for other vaccines.
 “This is an important development toward developing a vaccine against COVID-19. This research conducted by our experts in the UMSOM Center for Vaccine Development and Global Health has the potential for impacting millions of people in the world, as we urgently need a vaccine against this deadly illness,” said E. Albert Reece, MD, PhD, MBA, Executive Vice President for Medical Affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor and Dean, University of Maryland School of Medicine.
“This is an important development toward developing a vaccine against COVID-19. This research conducted by our experts in the UMSOM Center for Vaccine Development and Global Health has the potential for impacting millions of people in the world, as we urgently need a vaccine against this deadly illness,” said E. Albert Reece, MD, PhD, MBA, Executive Vice President for Medical Affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor and Dean, University of Maryland School of Medicine.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 45 academic departments, centers, institutes, and programs; and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.2 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically based care for nearly 2 million patients each year. The School of Medicine has more than $540 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 student trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of nearly $6 billion and an economic impact more than $15 billion on the state and local community. The School of Medicine faculty, which ranks as the 8th highest among public medical schools in research productivity, is an innovator in translational medicine, with 600 active patents and 24 start-up companies. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu.

About the Center for Vaccine Development and Global Health
For over 40 years, researchers in the Center for Vaccine Development and Global Health have worked domestically and internationally to develop, test, and deploy vaccines to aid the world’s underserved populations. CVD is an academic enterprise engaged in the full range of infectious disease intervention from basic laboratory research through vaccine development, pre-clinical and clinical evaluation, large-scale pre-licensure field studies, and post-licensure assessments. CVD has worked to eliminate vaccine-preventable diseases. CVD has created and tested vaccines against cholera, typhoid fever, paratyphoid fever, non-typhoidal Salmonella disease, shigellosis (bacillary dysentery), Escherichia coli diarrhea, nosocomial pathogens, tularemia, influenza, malaria, and other infectious diseases. CVD’s research covers the broader goal of improving global health by conducting innovative, leading research in Baltimore and around the world. CVD researchers are developing new and improved ways to diagnose, prevent, treat, control, and eliminate diseases of global impact. Currently, these diseases include typhoid, Shigella, E. coli diarrhea, malaria, and other vaccine-preventable infectious diseases. CVD researchers have been involved in critical vaccine development for emerging pathogens such as Ebola and Zika. In addition, CVD’s work focuses on the ever-growing challenge of antimicrobial resistance.
Contact
Joanne Morrison
Director of Marketing and Public Relations
Center for Vaccine Development and Global Health
University of Maryland School of Medicine
jmorrison@som.umaryland.edu
Office: (410) 706-2884
Mobile: (202) 841-3369