A Personalized Model of COQ2 Nephropathy Rescued by the Wild-Type COQ2 Allele for Dietary Coenzyme Q10 Supplementation
Zhu JY, Fu Y, Richman A, Zhao Z, Ray PE, and Han Z. J Am Soc Nephrol. 2017; 28(9):2607-2617.
In the news:
Supplement can lessen kidney damage linked to genetic mutations in transgenic fruit flies (American Association for the Advancement of Science (AAAS))
Research led by Zhe Han featured on cover of JASN, leading kidney disease journal (Children's National)
Patient clinical features:
The first sign of a kidney abnormality was abnormal amounts of urine production (severe oliguria) noted at five days of life in a male patient (Diomedi-Camassei et al, 2007). Renal biopsy at ten days, revealed severe extra capillary proliferation which rapidly developed into end-stage renal disease. At three months the patient developed drug-resistant progressive epileptic encephalopathy. MRI revealed cortical and subcortical stroke-like lesions. Ultimately the patient died at six months of age due to worsening into a state of unresponsiveness, hypotonia and respiratory failure. Ultrastructural examination of renal specimens showed an increased number of dysmorphic mitrochondria in glomerular cells. Biochemical analyses demonstrated decreased activities of respiratory chain complexes [II+III] and decreased CoQ10 concentrations in skeletal muscle and renal cortex.
Patient genetic variant:
Genomic DNA, purified from peripheral blood, was used in direct sequencing assays to screen for mutations in the nephrin and podocin genes (NPHS1 and NPHS2), and in the COQ2 and PDSS2 genes (Diomedi-Camassei et al, 2007). This lead to discovery of the patient carrying a homozygous c.437G3A (p.Ser146Asn) mutation in COQ2. Both parents were found to be heterozygous for the COQ2-S146N variant. Furthermore, this new mutation was not detected in 500 control chromosomes.
Footnote: Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, Ghiggeri GM, Murer L, Barisoni L, Pastore A, Muda AO, Valente ML, Bertini E, Emma F. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007; 18(10):2773-2780.
Precision Drosophila disease model:
We first examined the nephrocyte-specific silencing of the fly homologous gene Coq2 (Dot-Gal4;UAS-Coq2-RNAi) and found that Coq2 silencing significantly reduced nephrocyte function (Figure 1), providing gene-level validation for the human COQ2 gene in renal function. We then generated two UAS-based transgenic fly lines, one for the reference human COQ2 gene (Dot-Gal4;UAS-Coq2-RNAi;UAS-COQ2 transgenic flies) and one for the patient-specific allele COQ2-S146N (Dot-Gal4;UAS-Coq2-RNAi;UAS-COQ2(S146N) transgenic flies), generated using our gene replacement strategy. We found that the reference, but not the patient, allele was able to rescue the nephrocyte functional defect caused by Coq2-IR (Figure 1). Thereby, providing variant-level validation for the COQ2-S146N variant and established its kidney disease causal association.
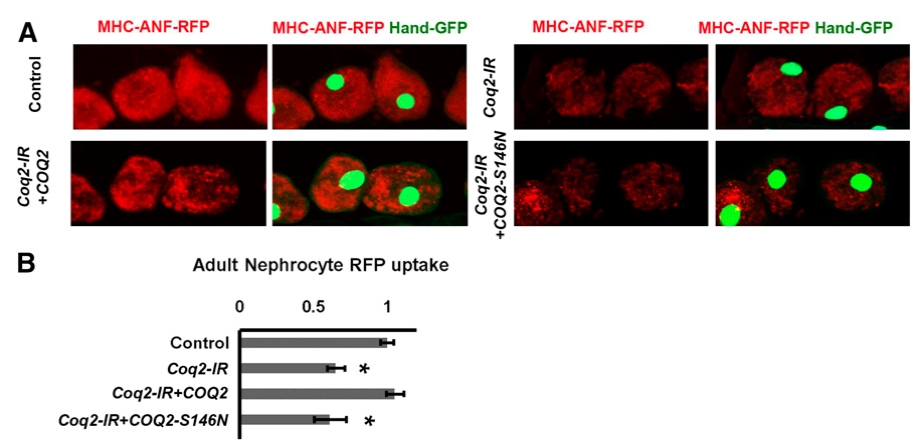
Figure 1. A normal allele of the human COQ2 gene but not a disease-variant allele (COQ2-S146A) rescued nephrocyte function in flies expressing Drosophila Coq2-IR. (A) Fluorescence micrographs showing uptake of ANF-RFP nephrocytes of 1-day postemergence adult flies. Left panels (MHC-ANF-RFP) show intracellular ANF-RFP fluorescence (red). Right panels (MHC-ANF-RFP Hand-GFP) show RFP (red) merged with GFP (green, mostly nuclear). Hand-GFP expression confirms pericardial nephrocyte cell identity. All flies are transgenic for Hand-GFP. Control flies carry the Dot-Gal4 driver but no RNAi construct. Coq2-IR flies carry Dot-Gal4 driving RNAi transgene silencing the endogenous Drosophila Coq2 gene expression, and where indicated, also a UAS-COQ2 (wild-type human COQ2 allele) or a UAS-COQ2-S146N (mutant human COQ2 allele).6 (B) Quantitation of RFP levels (expressed relative to control) in control versus Coq2-IR nephrocytes expressing normal COQ2 or mutant COQ2-S146N. For each genotype, 30 nephrocytes (six nephrocytes from each of five flies) were examined (*P,0.05).
The COQ2 gene encodes an enzyme in the Coenzyme Q10 pathway with Co-Q10 (Q10) as the final product.
Conveniently, Q10 is readily available as an over-the-counter dietary supplement, which motivated us to test Co-Q10 as a therapeutic strategy to treat patient with COQ2-renal disease. Indeed, supplementing the diet of Coq2-RNAi (Coq2-IR) flies with 5% Q10 rescued the nephrocyte functional phenotype (normal levels of ANF-RFP uptake by the nephrocytes), while we observed no differences in ANF-RFP uptake in wild-type controls flies that received supplemental Q10 in their diet (Figure 1, A and B). Furthermore, we showed that dietary treatment of 5% Co-Q10 largely restored normal slit diaphragm and lacunar channel morphology (Figure 2, C, D and E) and rescued the nephrocyte functional defect evident by reduced ROS levels in Coq2-IR nephrocytes after 5% Co-Q10 treatment (Figure 2, F and G). Moreover, Co-Q10 treatment also rescued the reduced lifespan of this personalized fly model (Figure 2).
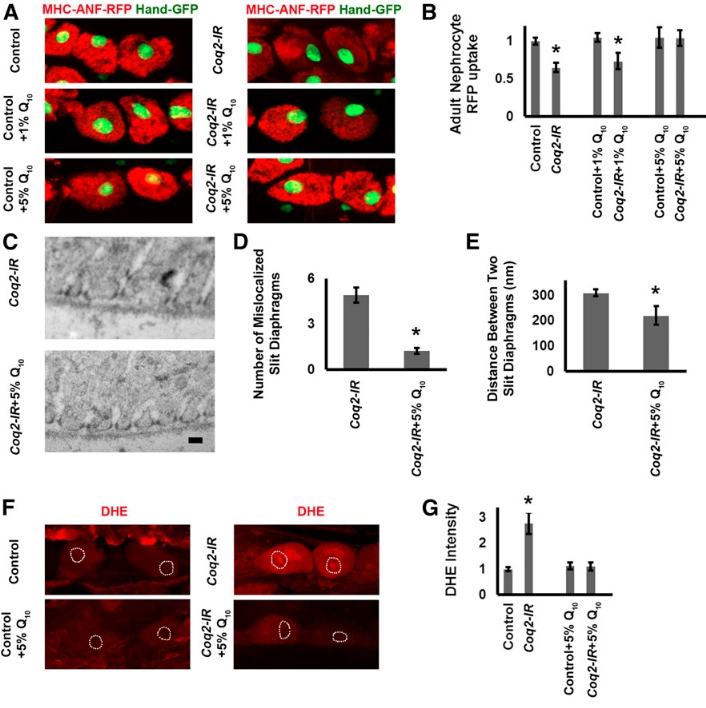
Figure 2. CoQ10 administration rescued nephrocyte functional and ultrastructural defects induced by Coq2 gene silencing. (A) Fluorescence micrographs showing nephrocytes of 1-day postemergence adult flies. Flies were reared from embryos on standard food supplemented with the indicated concentrations (1% or 5%) of CoQ10 (Q10). Left panels (MHC-ANF-RFP) show intracellular ANF-RFP fluorescence (red). Right panels (MHC-ANF-RFP Hand-GFP) show RFP (red) merged with GFP (green, mostly nuclear). A GFP transgene is expressed under the control of a Hand gene enhancer (Hand-GFP) to confirm pericardial nephrocyte cell identity. All flies are transgenic for Hand-GFP. Control flies carry the Dot-Gal4 driver but no RNAi construct. Coq2 flies carry Dot-Gal4 driving RNAi transgene silencing Coq2 expression. (B) Quantitation of RFP levels in control versus Coq2-IR nephrocytes (expressed relative to control) with no Q10, 1% Q10, or 5% Q10 dietary supplementation. For each genotype, 30 nephrocytes (six nephrocytes from each of five flies) were examined (* P < 0.05). (C) Transmission electron microscopy (TEM) showing mislocalized slit diaphragms and irregularly spaced and collapsed lacunar channel ultrastructure induced by Coq2 silencing (upper panel) rescued by administration of 5% Q10 (lower panel). Scale bar, 300 nm. (D) Quantitation of ectopic slit diaphragms in Coq2-IR versus Coq2-IR plus 5% Q10 supplementation nephrocytes. Average number of slit diaphragms positioned along interior channel membranes per 2,000 nm length of cell circumference (* P < 0.05). (E) The average distance (in nm) between normally localized slit diaphragms in Coq2-IR versus Coq2-IR plus 5% Q10 supplementation nephrocytes (* P < 0.05). (F) Reactive oxygen species (ROS) levels in normal (control) and Coq2-silenced (Coq2-IR) nephrocytes were indicated by oxidized dihydroethidium (DHE), a redox indicator, red fluorescence in the cell nucleus. ROS levels were higher in Coq2-IR nephrocytes, and feeding Coq2-IR larvae a diet supplemented with 5% Q10 reduced nephrocyte ROS levels. Dashed lines indicate cell nuclei (determined from DAPI staining, not shown). (G) Levels of DHE red nuclear fluorescence expressed relative to normal control larval nephrocytes fed a non-supplemented diet. Coq2 gene silencing led to a 2.5–3-fold increase in ROS levels. Feeding Coq2-IR 5% Q10 lowered ROS to normal levels. In each case, 30 nephrocytes (six nephrocytes from each of five larvae) were examined (* P < 0.05).
Our findings thus demonstrate that a patient derived mutation contributing to the development of a specific glomerular disease can be introduced into our Drosophila model, in effect creating a personalized approach to investigate the pathogenesis and even treatment of specific renal diseases in vivo.
