Our Mission
The Preclinical Section is tasked to provide clinical and translational expertise to the Center’s pipeline projects in interpreting medical records and translating scientific discoveries using Precision Disease models to personalized medicine treatment that benefit patients. The Preclinical Section also serves as a vital bridge between patient-derived clinical and genomic data and precision animal modeling, ensuring the clinical relevance and translational impact of the Center’s activities.
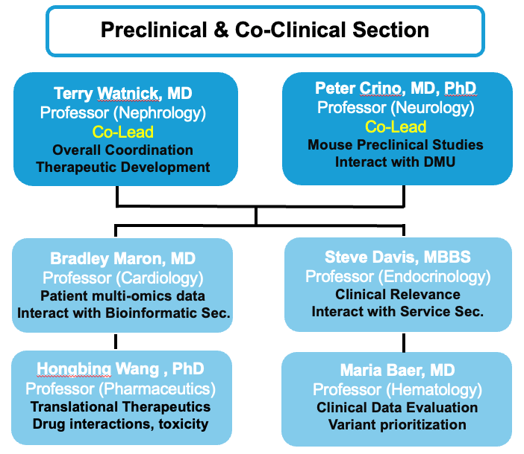
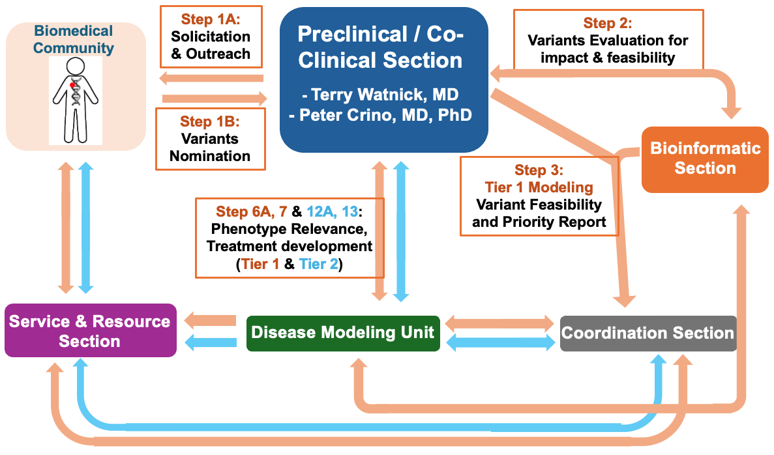
Roles of the Preclinical Section and its interaction with the other center sections
Organizational Chart