Artery/Vein Specification Is Governed by Opposing Phosphatidylinositol-3 Kinase and MAP Kinase/ERK Signaling
Hong CC, Peterson QP, Hong J-Y, and Peterson RT. Curr Biol. 2006; 16(13):1366-72.
Zebrafish disease model:
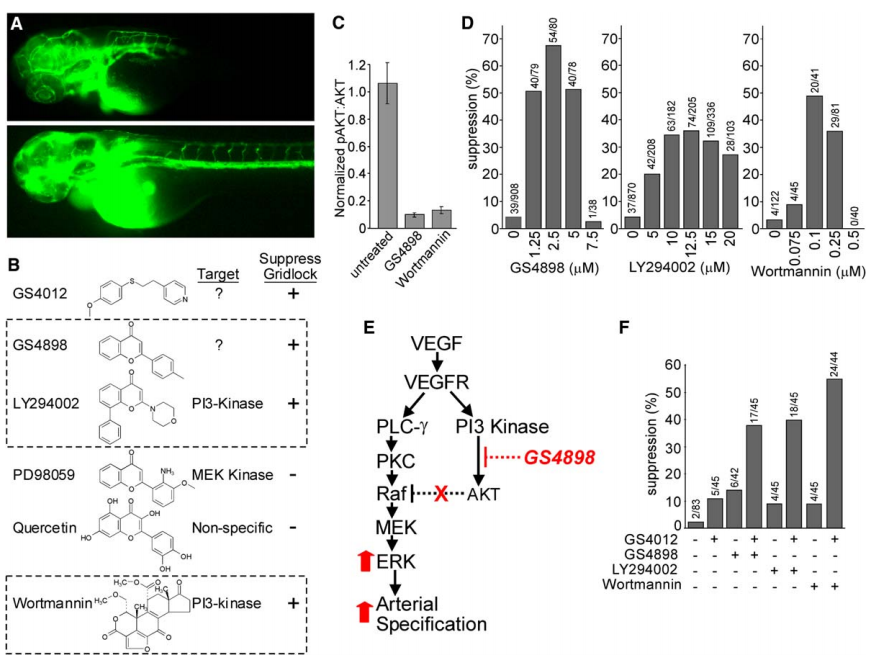
Zebrafish with a mutation (grlm145) in the “gridlock” gene lack trunk and tail circulation because of an aortic dysmorphogenesis that resembles congenital aortic coarctation in humans (Figure 1A). Beyond gridlock, other genetic and biochemical factors are likely involved in artery versus vein (A/V) specification, but identifying additional factors via traditional genetic approaches may be difficult, particularly if such factors are necessary for vital biological processes that occur prior to the formation of the vasculature. For the identification of novel factors that govern the artery/vein cell fate decision, it is important to employ a strategy that enables control over the dose and timing of gene inactivation. Therefore, instead of traditional genetic approaches, we employed small-molecule screening to identify conditional modifiers of the artery/vein cell fate decision. We identified that a small molecular compound, GS4898, is capable of suppressing the congenital aortic coarctation-like phenotype in the 1–5 mM range (Figure 1D). In addition, we show that activation of ERK (p42/44 MAP kinase) is a specific marker of early arterial progenitors and is among the earliest known determinants of the arterial specification. In embryos, cells fated to contribute to arteries express high levels of activated ERK, whereas cells fated to contribute to veins do not. Inhibiting the phosphatidylinositol-3 kinase (PI3K) branch with GS4898 or known PI3K inhibitors, or by expression of a dominant-negative form of AKT promotes arterial specification.