Friday, April 30, 2021
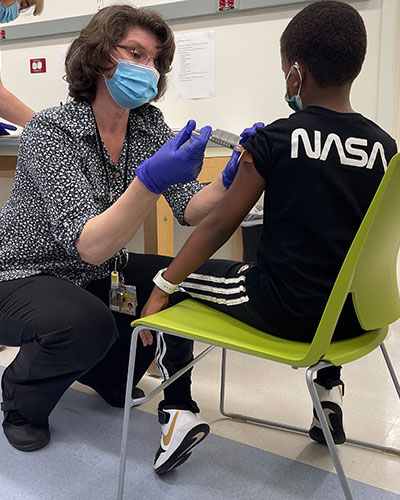
University of Maryland School of Medicine Begins Pediatric Trial of Moderna COVID-19 Vaccine
This Phase 2/3 Trial Will Test the Vaccine in Children Ages 6 Months to 11 Years

Friday, February 05, 2021
UM School of Medicine Researchers Demonstrate Strong Immune Response for New COVID-19 Vaccine in Pre-Clinical Tests
Researchers at the University of Maryland School of Medicine (UMSOM) have found promising results in pre-clinical studies for a new experimental vaccine against COVID-19 made by Novavax. The vaccine was found to generate a robust immune response in animals exposed to the vaccine with strong data indicating safety and efficacy, according to the study published recently in the journal Nature Communications. The results have been used to begin testing the vaccine in human trials in the U.S. with a Phase 3 trial that recently launched at the UMSOM’s Center for Vaccine Development and Global Health.

Wednesday, January 27, 2021
Dr. Wilbur Chen, Nationally-Recognized Vaccine Researcher, Selected for Federal Committee that Guides Immunization Policies
Wilbur H. Chen, MD, MS, FIDSA, FACP, Professor of Medicine at the University of Maryland School of Medicine (UMSOM), has been named a new voting member of the federal government’s Advisory Committee on Immunization Practices (ACIP), the prestigious board of experts that makes recommendations on the safe use of vaccines for Americans. The U.S. Department of Health and Human Services selected Dr. Chen for the 15-member advisory committee based on his expertise and national leadership in vaccinology, infectious diseases, public health, and preventive medicine. He will remain in his current role at UMSOM while he serves in his four-year term, which began last month.