News

August 1, 2022
A new study reveals the role of extracellular RNA sensing in acute respiratory distress syndrome in sepsis
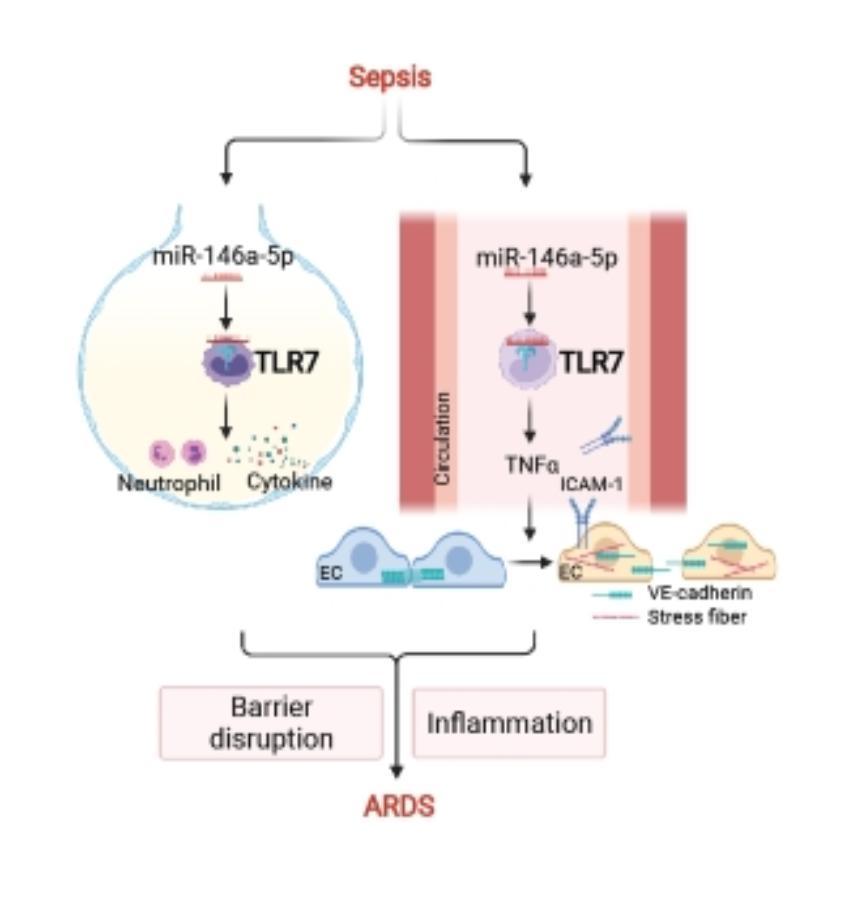
Sepsis-induced acute respiratory distress syndrome (ARDS) causes profound morbidity and mortality worldwide. Pulmonary inflammation and endothelial barrier disruption are pathological hallmarks. A team of scientists led by Dr. Lin Zou from the School of Medicine and Pharmacy of the University of Maryland investigated the role of nucleic acid sensing in these pathological changes. They discovered that intratracheal delivery of microRNA (miR)-146-5p triggered lung inflammation and disrupted alveolar barrier function via Toll-like receptor 7 (TLR7), a single-stranded RNA sensor. Systemic deficiency of TLR7 markedly reduced sepsis-associated lung injury manifested as decreased bronchoalveolar lavage and lung tissue cytokines and preserved pulmonary barrier function. Surprisingly, endothelial injury mediated by the miR-146a-5p-TLR7 pathway appeared attributed to an indirect mechanism likely mediated by miR-146a-5p-TLR7 sensing in immune cells through gap junction proteins VE-cadherin. Finally, cytokine array, pathway enrichment analysis, and neutralizing antibody experiments identified TNFa as the key downstream effector of miR-146a-5p-TLR7 signaling responsible for endothelial injury (See Figure). These findings are recently published in Am J Respir Cell Mol Biol.
Figure:

November 19, 2021
microRNA-TLR7 Signaling Mediates Brain Inflammation during Bacterial Sepsis
Sepsis is a serious clinical condition with organ dysfunction mediated by a dysregulated host response to infection. Up to 70% of septic patients develop cerebral dysfunction, a condition termed sepsis-associated encephalopathy (SAE). Septic patients with SAE have a higher mortality rate than patients with normal mental status. Among sepsis survivors, many developed long-term cognitive impairment that adversely impacts the quality of their lives. The molecular mechanism underlying SAE is not well understood. A multidisciplinary research team led by Lin Zou, Junfang Wu, Wei Chao at the Center for Shock, Trauma and Anesthesiology Research reported an important role of microRNA®TLR7 sensing in brain innate immune response in murine sepsis. Mice with SAE exhibited brain-blood barrier (BBB) disruption, profound neuroinflammation, and motor coordination, and neurological impairment. Sepsis significantly increased plasma miRNA levels, such as miR-146a-5p. Exogenous miR-146a-5p induces innate immune responses in both cultured microglia/astrocytes and the intact brain via a TLR7-dependent manner. Moreover, miR-146a-/- mice showed reduced accumulation of monocytes and neutrophils in the brain compared to WT after sepsis. Finally, TLR7-/- mice had preserved BBB integrity, reduced microglial expansion, and leukocyte accumulation in the brain, but did not improve neurobehavioral recovery following sepsis. These data establish an important role of extracellular miRNA and TLR7 sensing in sepsis-induced brain inflammation. The study is published online in Brain, Behavior, and Immunity.

November 12, 2021
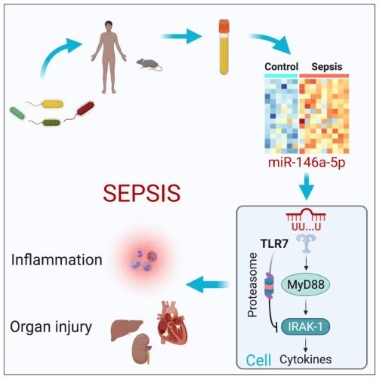
A new study reveals a critical role of extracellular microRNA in innate immunity and sepsis

September 9, 2021
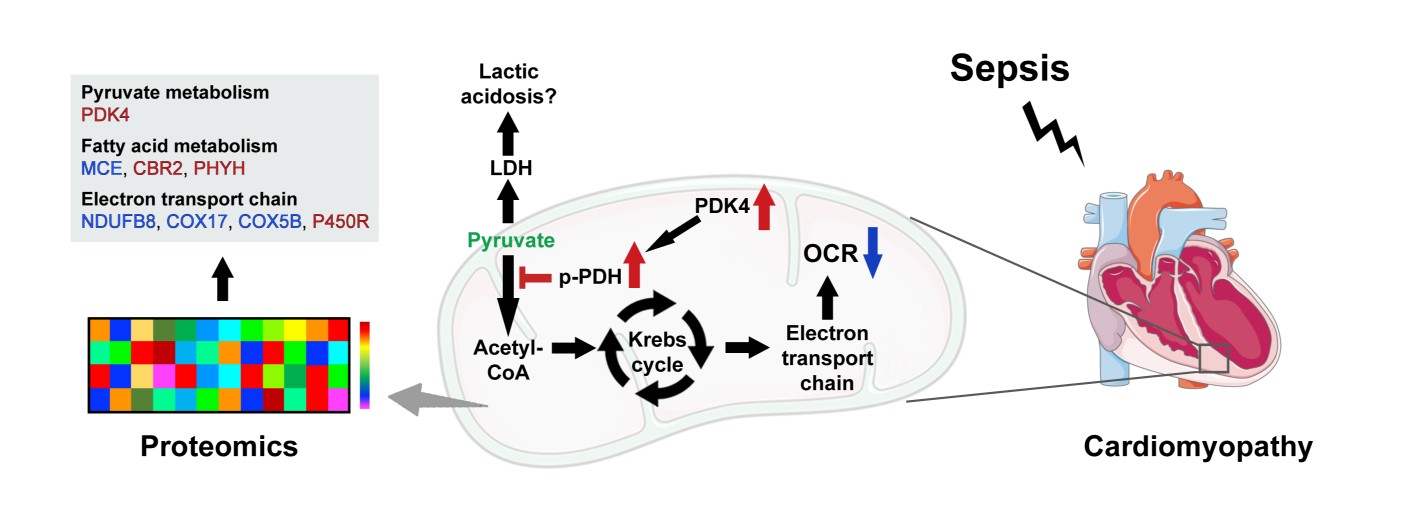
Dissecting mitochondrial metabolic dysfunction linked to sepsis-induced cardiomyopathy

Figure 1:

Lin Zou, MD, PhD
July 01, 2021 - Congratulations to Lin Zou MD, PhD on her promotion to Associate Professor with the University of Maryland, School of Medicine; Department of Anesthesiology.

Wei Chao, MD, PhD and Lin Zou, MD, PhD
2019- Wei Chao, MD, PhD, Junfang Wu, PhD, and Lin Zou, MD, PhD R01 funded grant titled "Targeting Brain Inflammation and Neurocognitive Dysfunction in Sepsis" from the National Institute of Neurological Disorders and Stroke. Click Here for more information.

Lin Zou, MD, PhD
2017- Lin Zou, MD, PhD received R35 funded grant titled "Function and Mechanisms of extracellular microRNAs in sepsis-induced lung injury" from the National Institute of General Medical Sciences. Click Here for more information.

Lin Zou, MD, PhD
2017- Lin Zou, MD, PhD awarded the prestigious Faculty Research Award from the Shock Society. The award is given in support of a faculty member at his/her early career stage to develop research in the areas related to trauma, shock, and sepsis, and who has the intent to become an outstanding independent investigator.
